Scalable in vitro production of defined mouse erythroblasts
- PMID: 34995303
- PMCID: PMC8741028
- DOI: 10.1371/journal.pone.0261950
Scalable in vitro production of defined mouse erythroblasts
Abstract
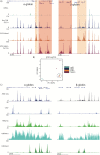
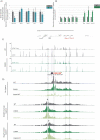
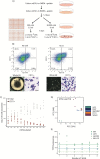
Mouse embryonic stem cells (mESCs) can be manipulated in vitro to recapitulate the process of erythropoiesis, during which multipotent cells undergo lineage specification, differentiation and maturation to produce erythroid cells. Although useful for identifying specific progenitors and precursors, this system has not been fully exploited as a source of cells to analyse erythropoiesis. Here, we establish a protocol in which characterised erythroblasts can be isolated in a scalable manner from differentiated embryoid bodies (EBs). Using transcriptional and epigenetic analysis, we demonstrate that this system faithfully recapitulates normal primitive erythropoiesis and fully reproduces the effects of natural and engineered mutations seen in primary cells obtained from mouse models. We anticipate this system to be of great value in reducing the time and costs of generating and maintaining mouse lines in a number of research scenarios.
Conflict of interest statement
D.M.J. is co-founder of Nucleome Therapeutics. The other authors declare no competing interests.
Figures


References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

