Relative Bioavailability and Food Effect of GSK3640254 Tablet and Capsule Formulations in Healthy Participants
- PMID: 34995417
- PMCID: PMC9306620
- DOI: 10.1002/cpdd.1051
Relative Bioavailability and Food Effect of GSK3640254 Tablet and Capsule Formulations in Healthy Participants
Abstract
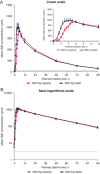
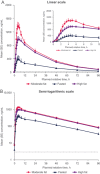
GSK3640254 is a next-generation maturation inhibitor with demonstrated potency across HIV-1 subtypes and a high barrier to emergent resistance. This phase I, 2-part, randomized, open-label study (ClinicalTrials.gov identifier, NCT04263142) in healthy participants assessed the relative bioavailability of a single dose of GSK3640254 200 mg in tablet and capsule formulations (part 1) and the effect of food on the pharmacokinetic profile of the tablet formulation (part 2). Overall, 39 participants were randomized to treatment (part 1, n = 18; part 2, n = 21). All participants in part 1 completed the study; 2 participants in part 2 withdrew before study completion (adverse event, n = 1; physician decision, n = 1). In part 1, plasma exposures of the GSK3640254 tablet formulation were not meaningfully different from those of the capsule formulation when administered in the presence of a moderate-fat meal. In part 2, GSK3640254 plasma exposures increased by ≈3- to 4-fold under high- and moderate-fat conditions, respectively, compared with fasted conditions. No major safety or tolerability findings were observed. The highest incidence of adverse events (24%) was reported under high-fat conditions. Taken together, these data support the use of the tablet formulation coadministered with food in the clinical development of GSK3640254 for treatment of HIV-1.
Keywords: GSK3640254; food effect; maturation inhibitor; pharmacokinetics; relative bioavailability.
© 2022 ViiV Healthcare. Clinical Pharmacology in Drug Development published by Wiley Periodicals LLC on behalf of American College of Clinical Pharmacology.
Conflict of interest statement
M.J., S.R.J., and M.L. are employees of ViiV Healthcare and may own stock in GlaxoSmithKline (GSK). A.M., V.B., and J.Z. are employees of and may own stock in GSK. T.P.D. was an employee of GSK during the conduct of the study and may own stock in GSK.
Figures

References
-
- Cihlar T, Fordyce M. Current status and prospects of HIV treatment. Curr Opin Virol. 2016;18:50‐56. - PubMed
-
- Morales‐Ramirez J, Bogner JR, Molina J‐M, et al. Safety, efficacy, and dose response of the maturation inhibitor GSK3532795 (formerly known as BMS‐955176) plus tenofovir/emtricitabine once daily in treatment‐naive HIV‐1‐infected adults: week 24 primary analysis from a randomized phase IIb trial. PLoS One. 2018;13(10):e0205368. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

