Molecular Probes, Chemosensors, and Nanosensors for Optical Detection of Biorelevant Molecules and Ions in Aqueous Media and Biofluids
- PMID: 34995461
- PMCID: PMC8832467
- DOI: 10.1021/acs.chemrev.1c00746
Molecular Probes, Chemosensors, and Nanosensors for Optical Detection of Biorelevant Molecules and Ions in Aqueous Media and Biofluids
Abstract
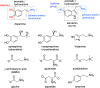
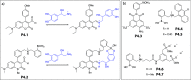
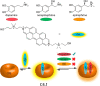
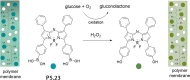
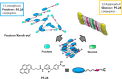
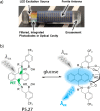
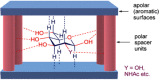
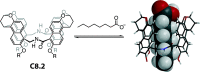
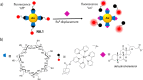
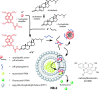
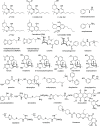
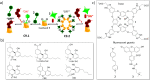
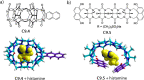
Synthetic molecular probes, chemosensors, and nanosensors used in combination with innovative assay protocols hold great potential for the development of robust, low-cost, and fast-responding sensors that are applicable in biofluids (urine, blood, and saliva). Particularly, the development of sensors for metabolites, neurotransmitters, drugs, and inorganic ions is highly desirable due to a lack of suitable biosensors. In addition, the monitoring and analysis of metabolic and signaling networks in cells and organisms by optical probes and chemosensors is becoming increasingly important in molecular biology and medicine. Thus, new perspectives for personalized diagnostics, theranostics, and biochemical/medical research will be unlocked when standing limitations of artificial binders and receptors are overcome. In this review, we survey synthetic sensing systems that have promising (future) application potential for the detection of small molecules, cations, and anions in aqueous media and biofluids. Special attention was given to sensing systems that provide a readily measurable optical signal through dynamic covalent chemistry, supramolecular host-guest interactions, or nanoparticles featuring plasmonic effects. This review shall also enable the reader to evaluate the current performance of molecular probes, chemosensors, and nanosensors in terms of sensitivity and selectivity with respect to practical requirement, and thereby inspiring new ideas for the development of further advanced systems.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
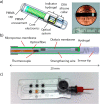
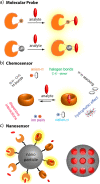



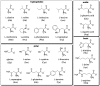
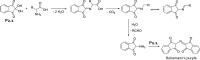

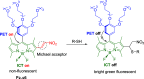
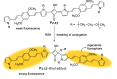
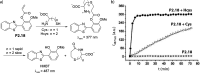
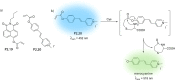



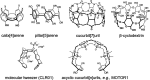
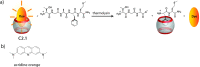


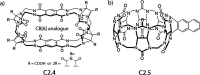


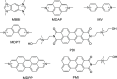

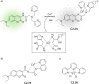
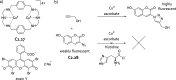
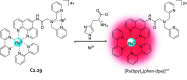
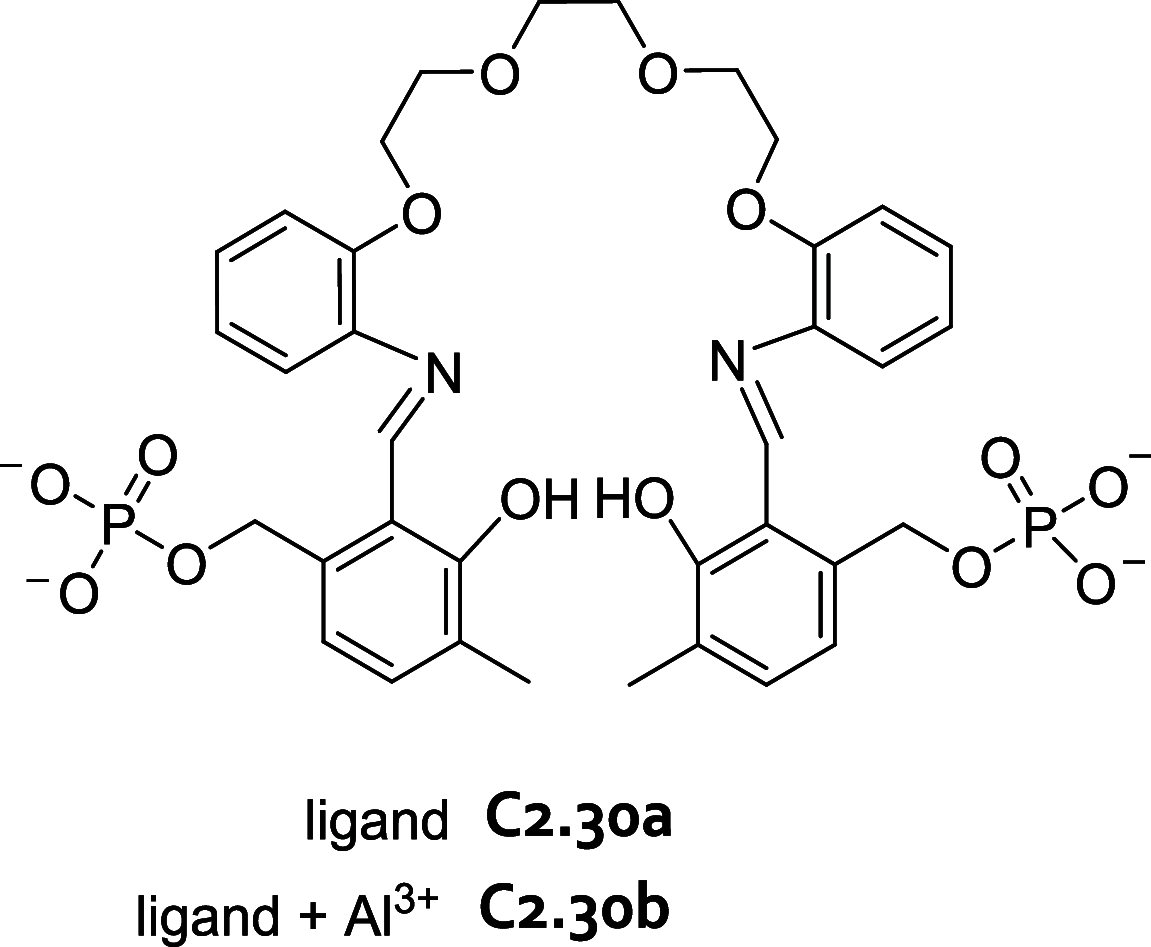

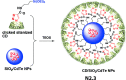
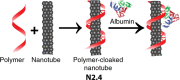
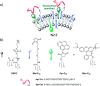
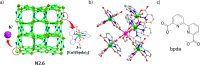
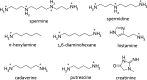
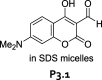
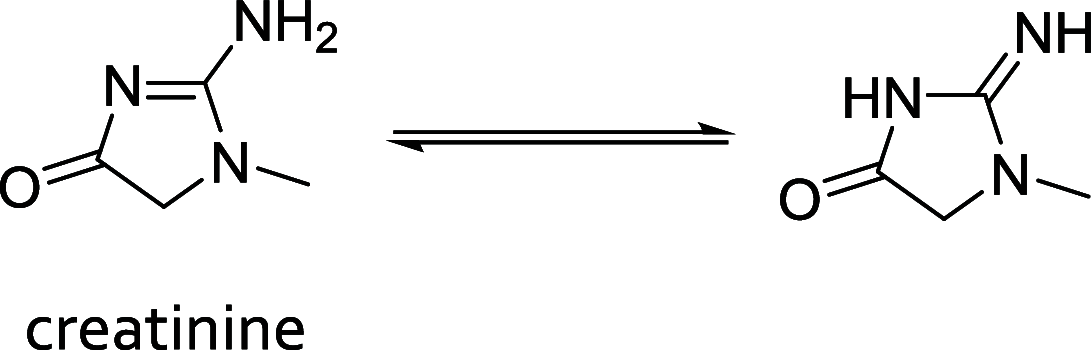



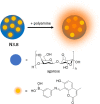

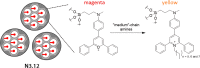
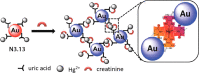
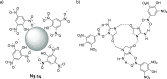
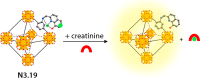
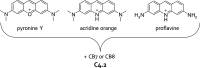

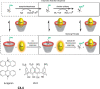
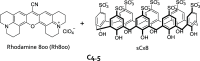
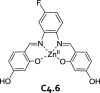

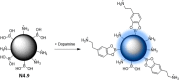
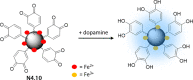
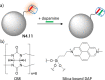
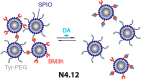




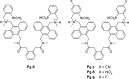
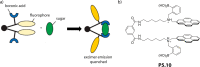
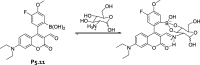
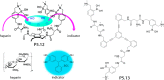
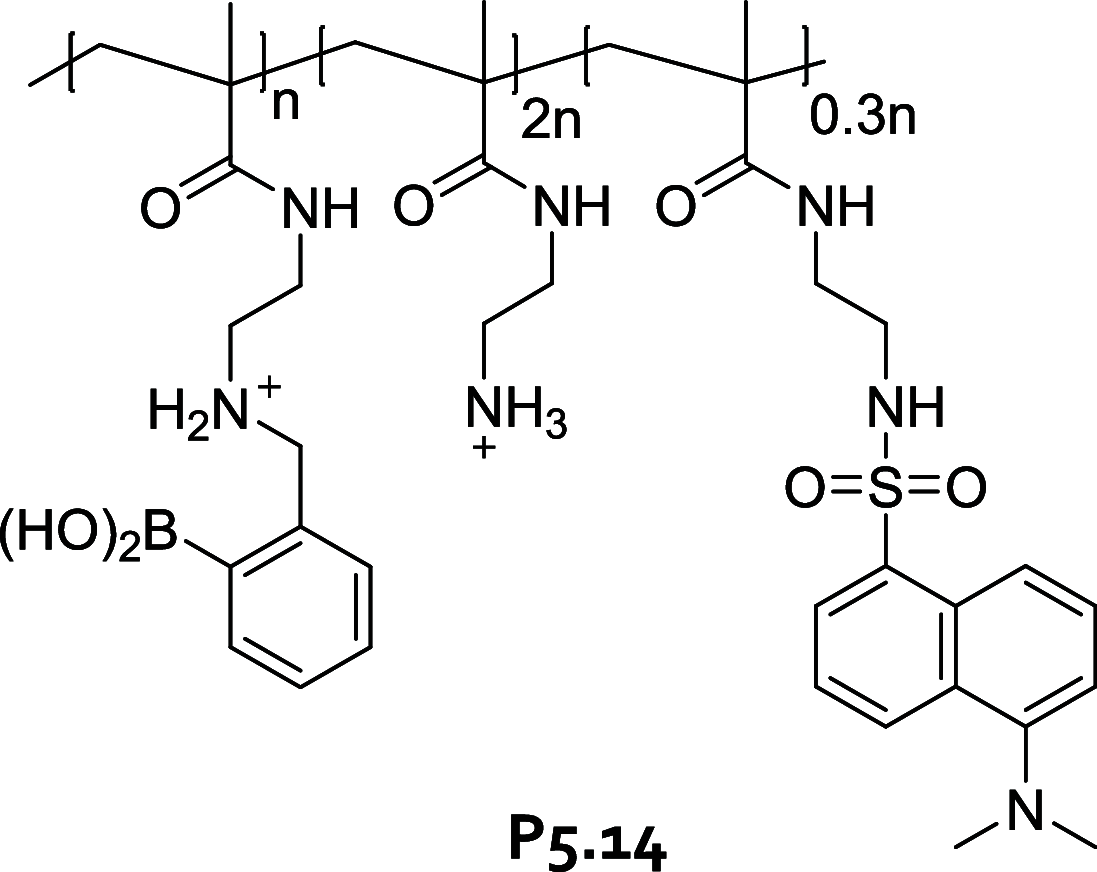

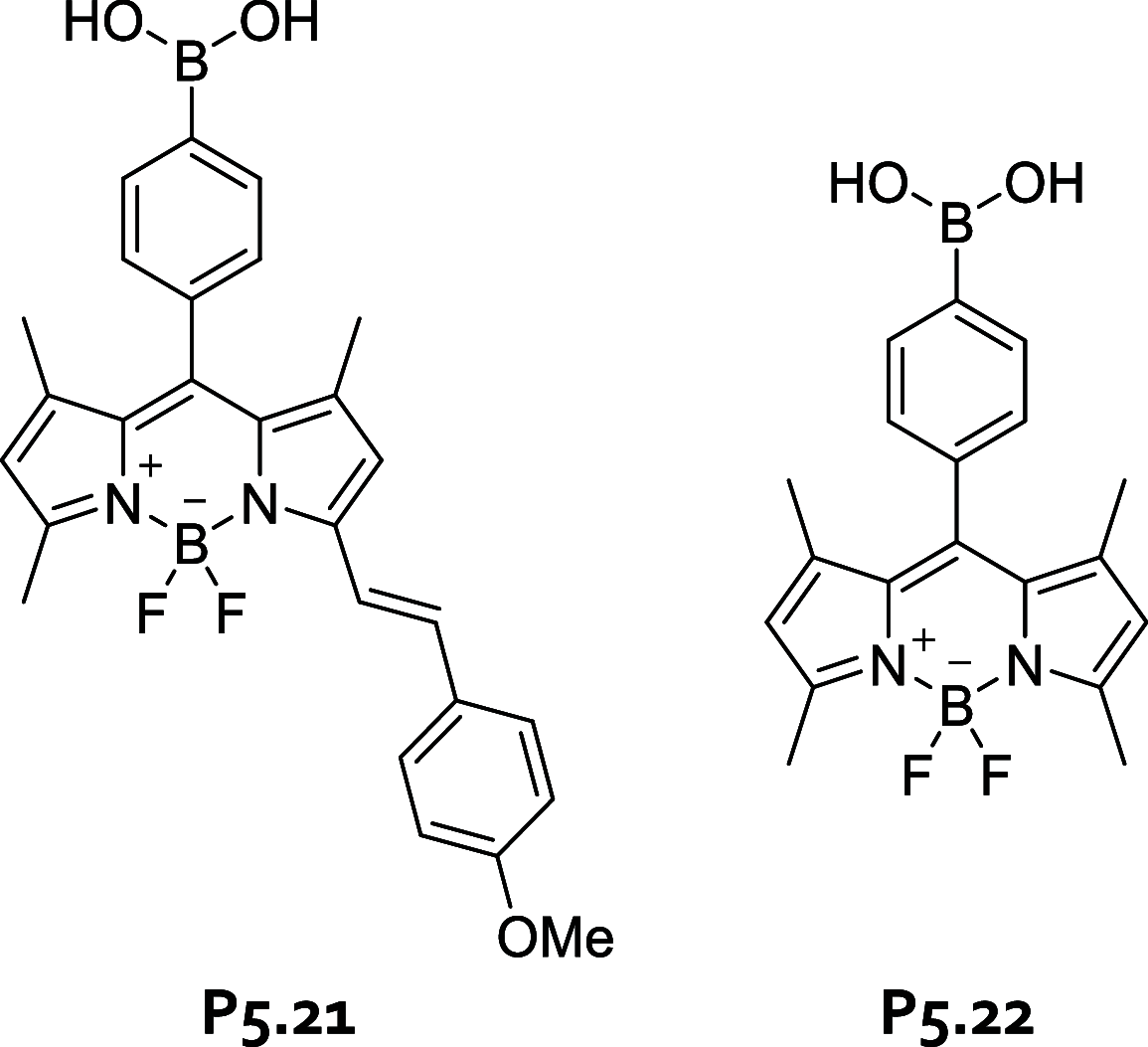



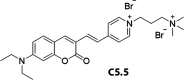
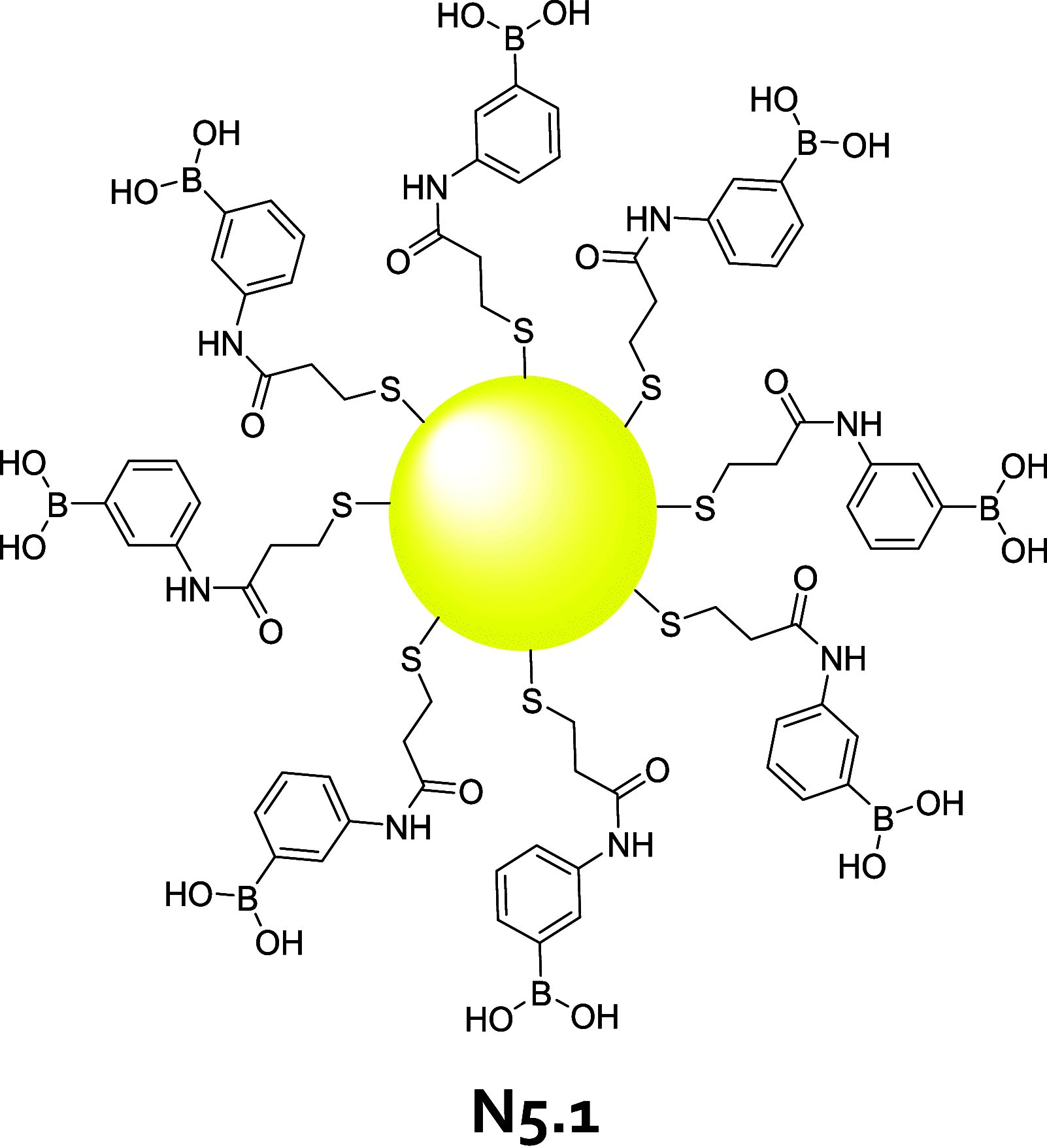


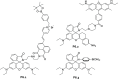
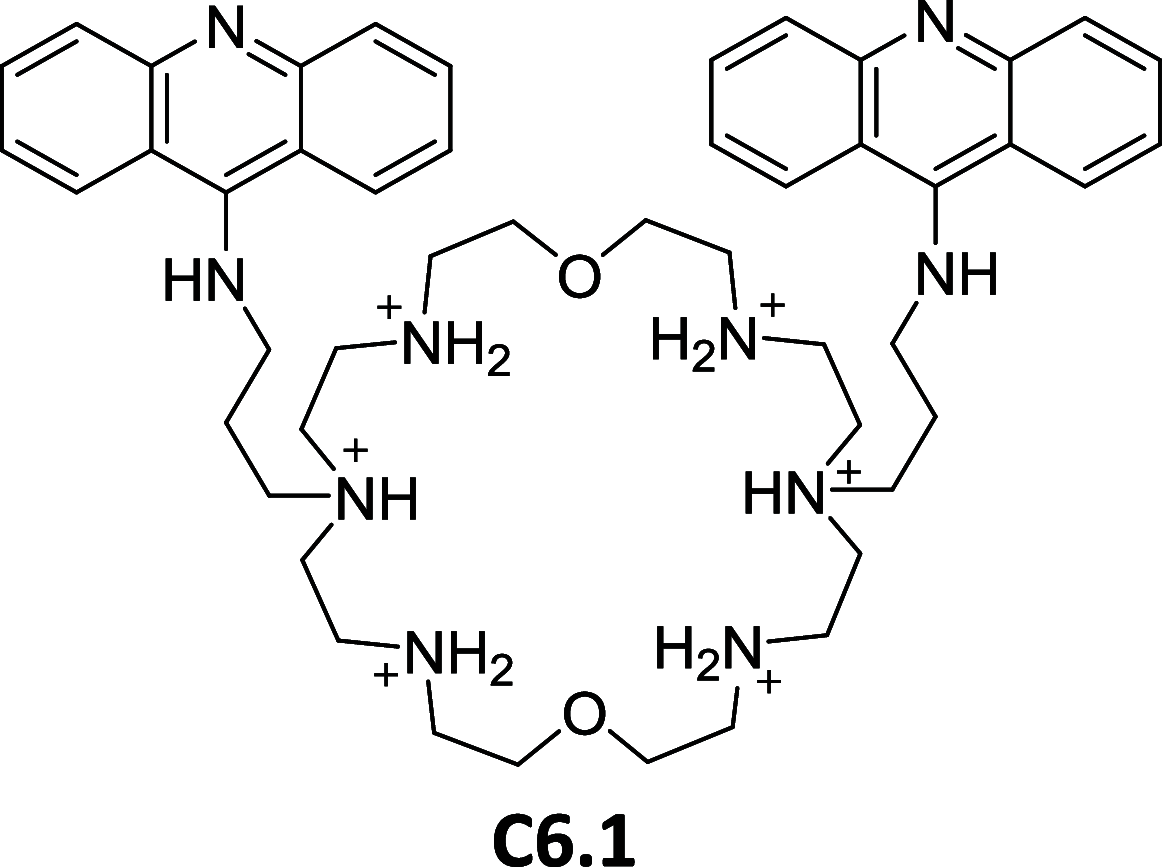
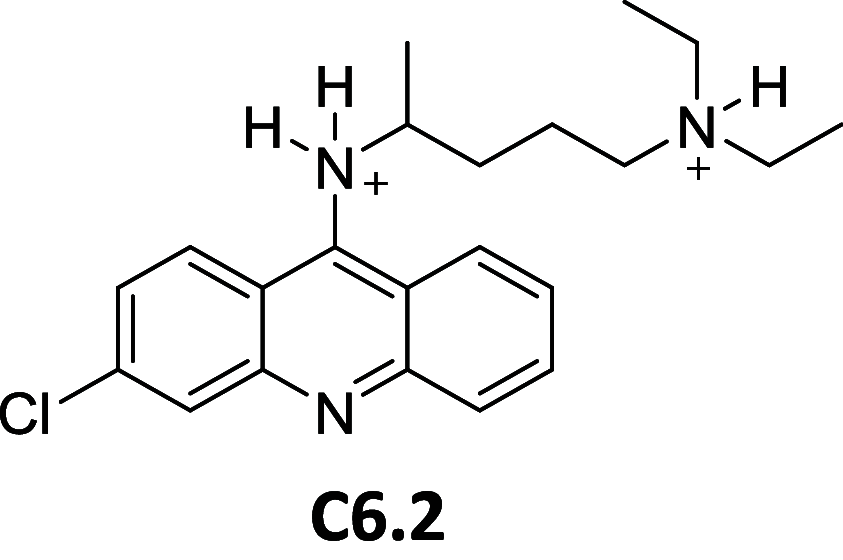



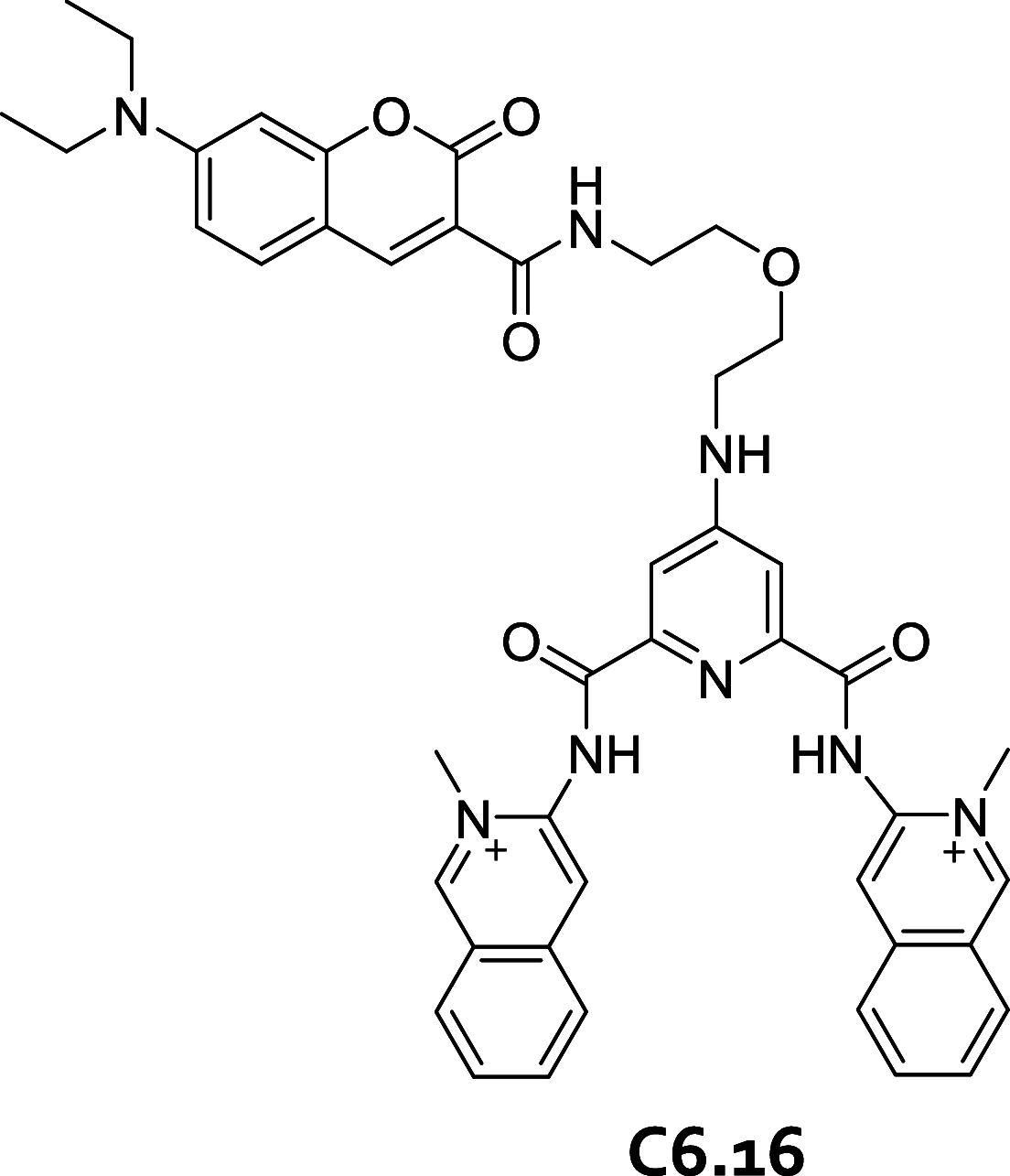
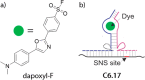
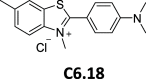
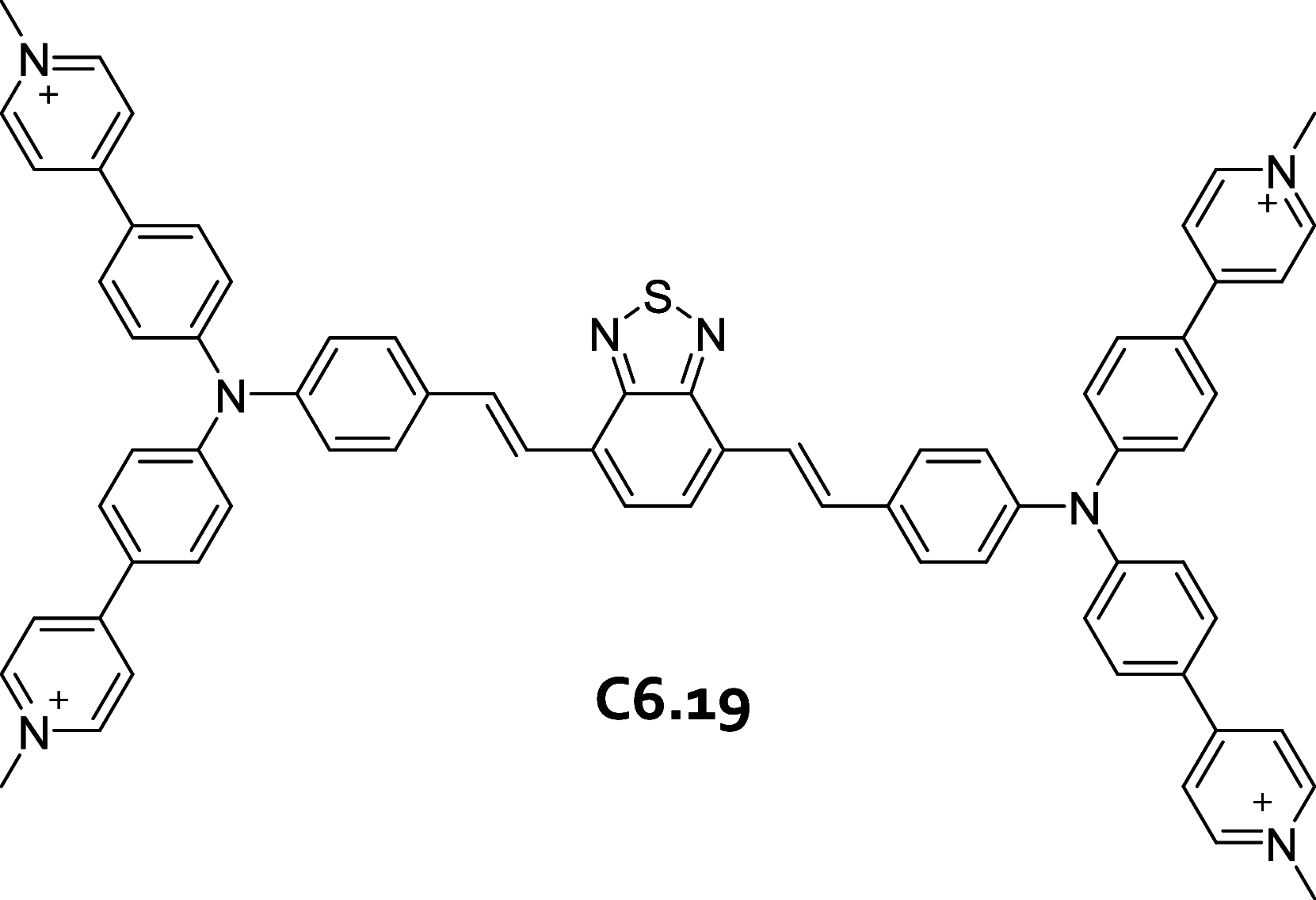

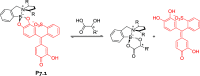
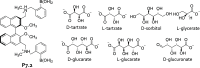
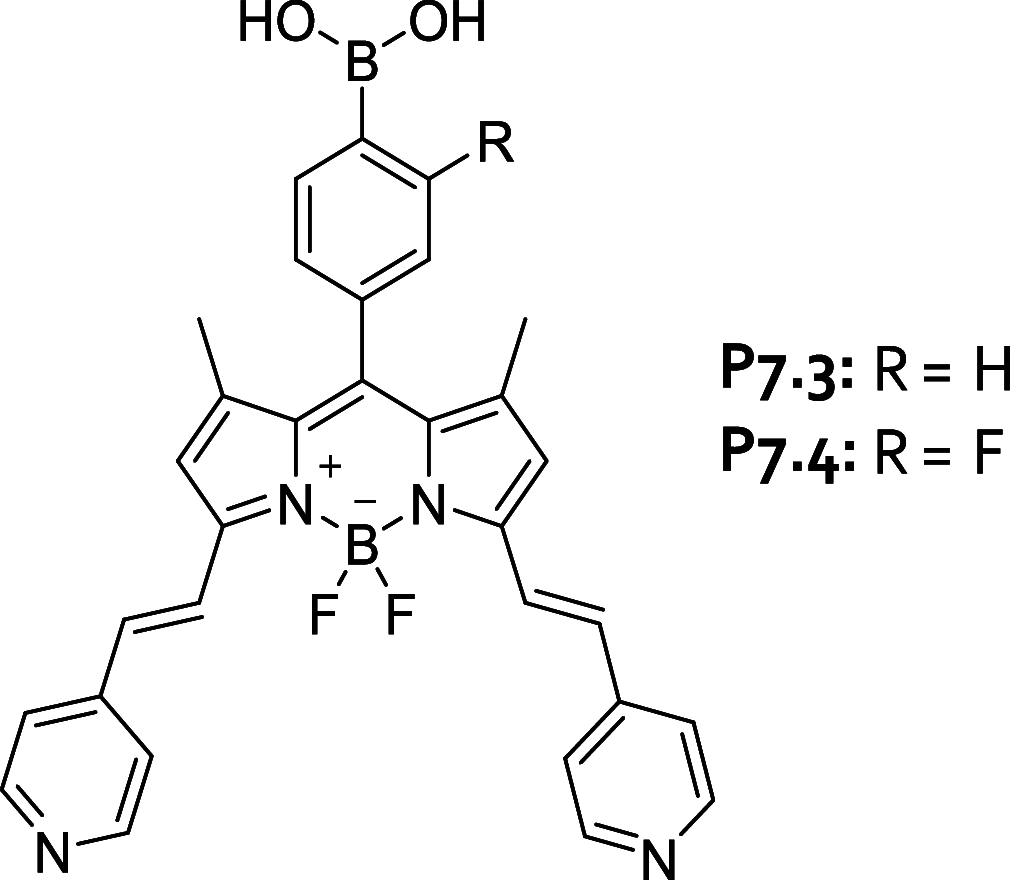
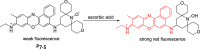
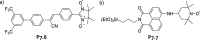
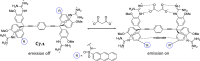
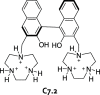
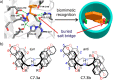
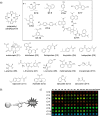
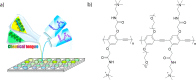
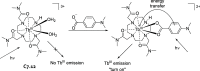
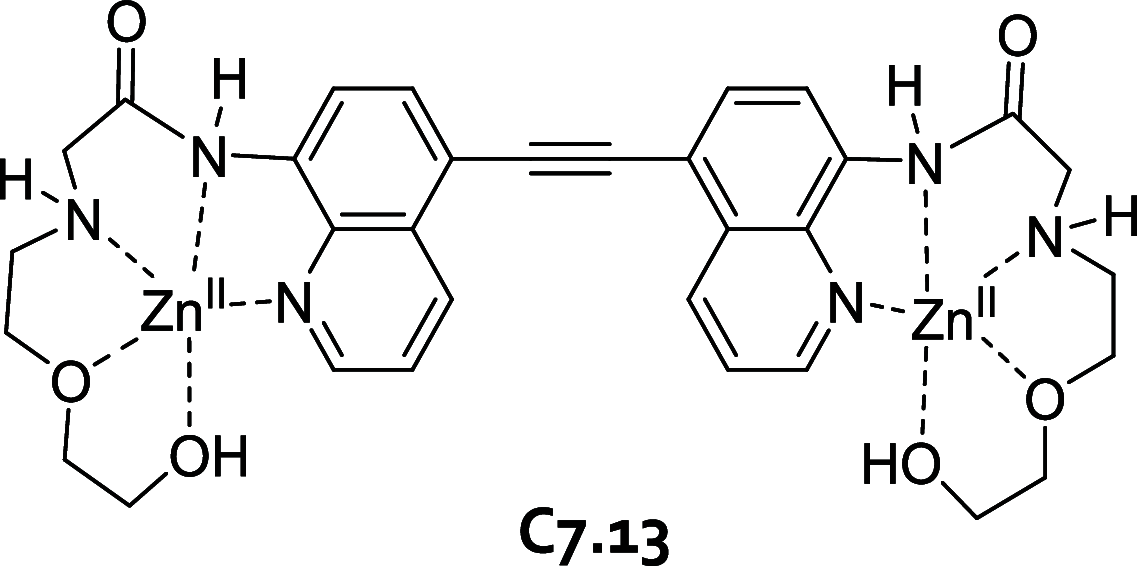

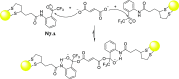
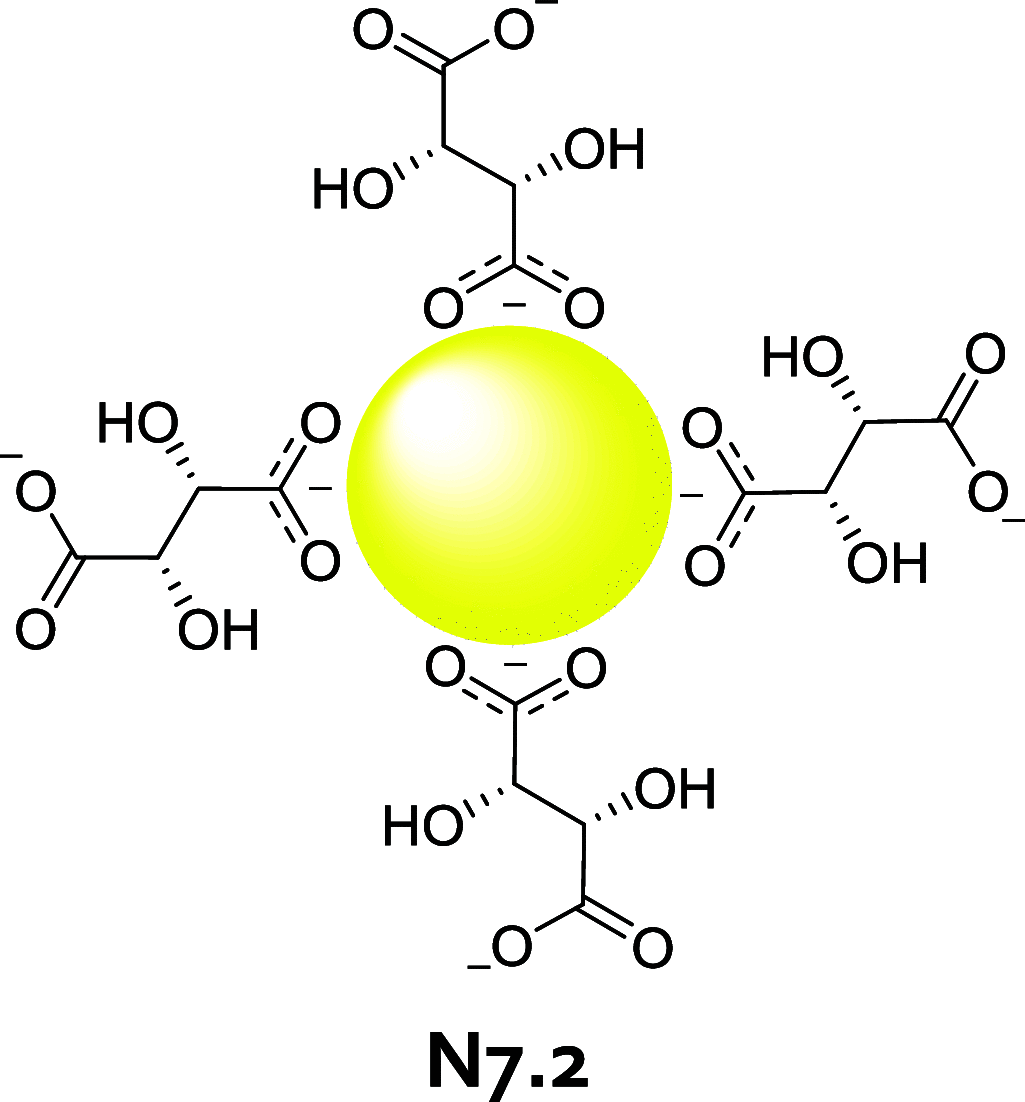
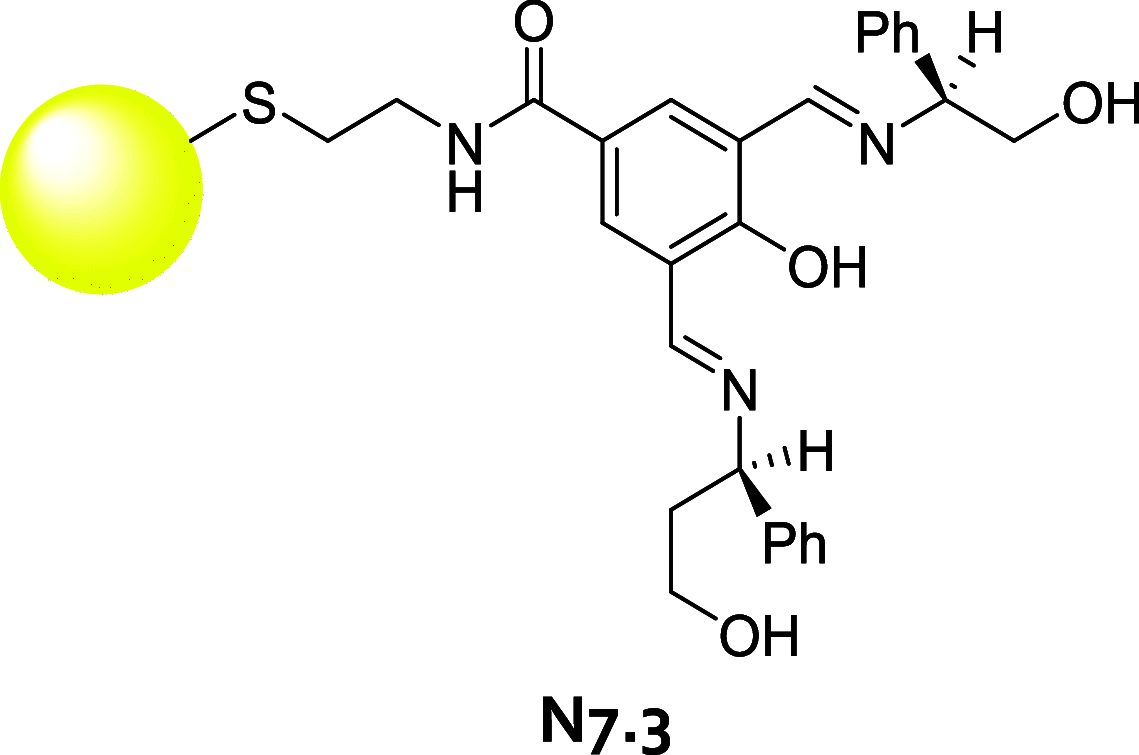





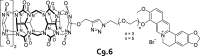
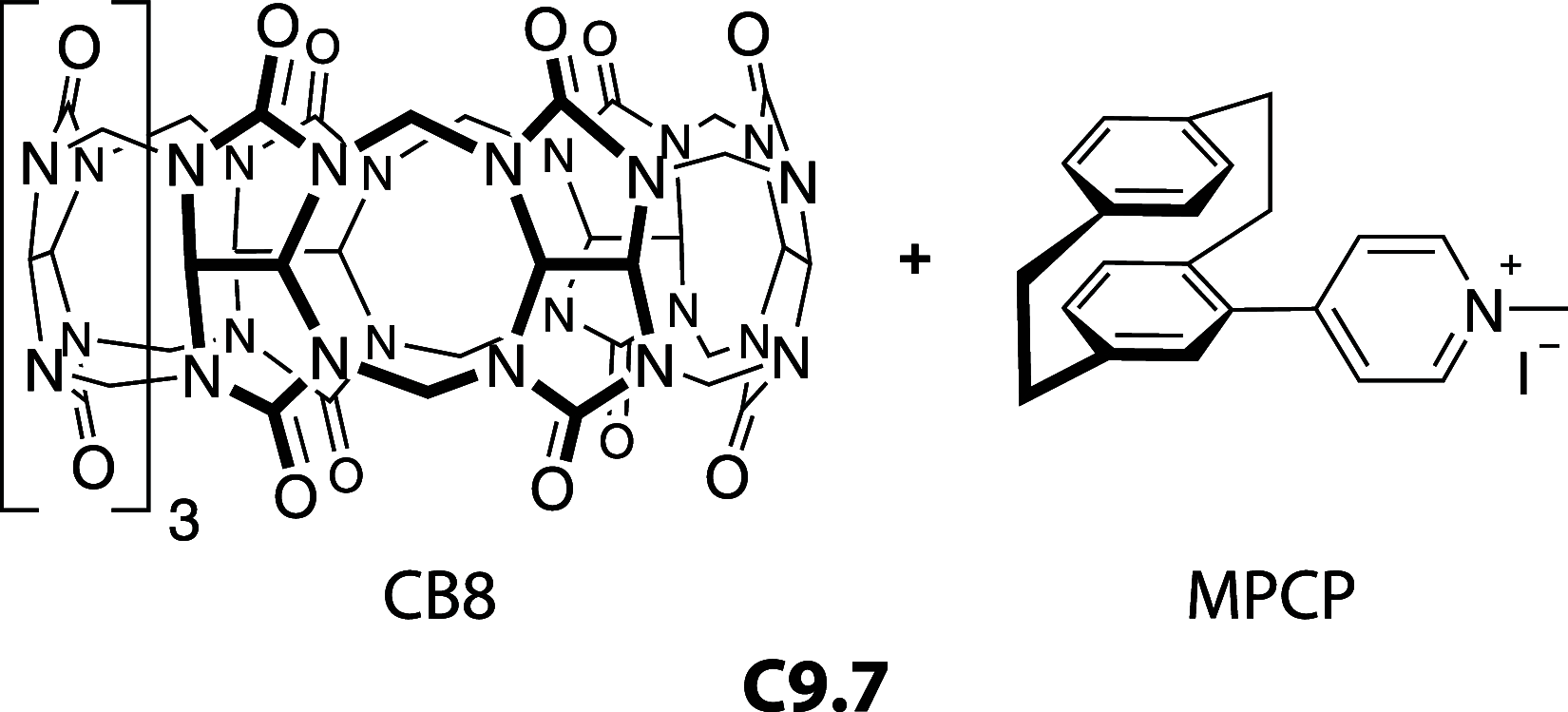
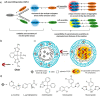
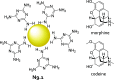
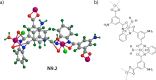

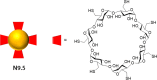

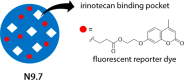
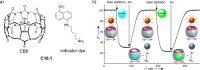
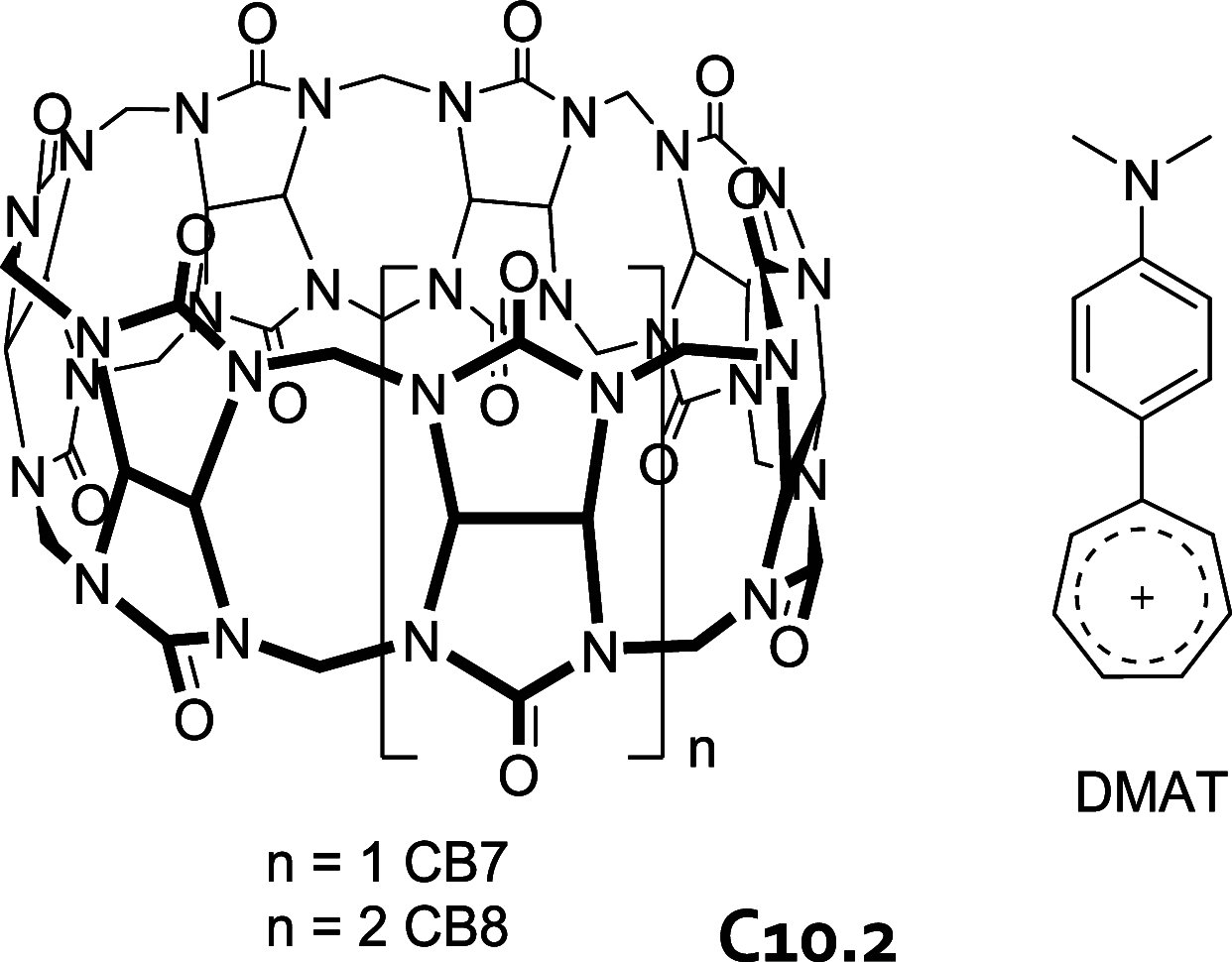

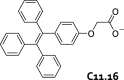
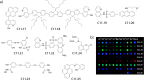
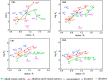
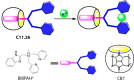


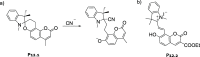
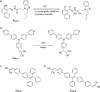
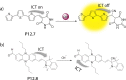
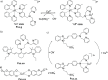

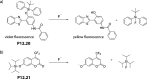
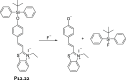
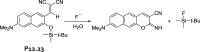
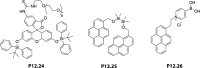
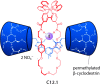
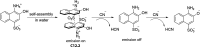
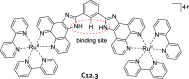
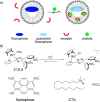
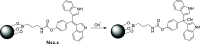
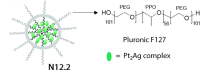


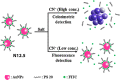
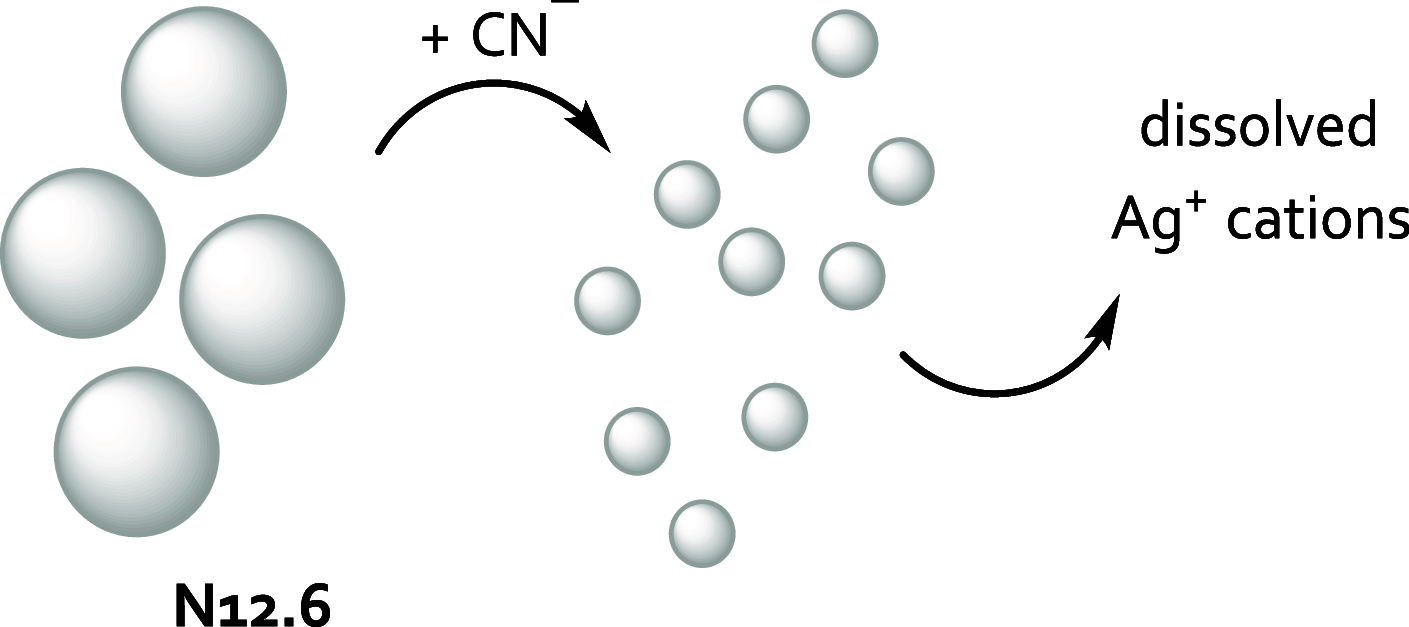
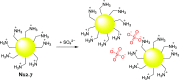
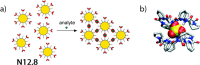
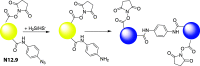
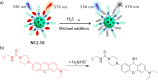
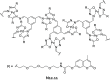
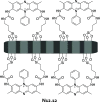
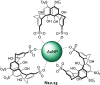

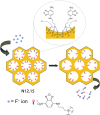
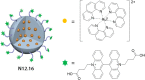
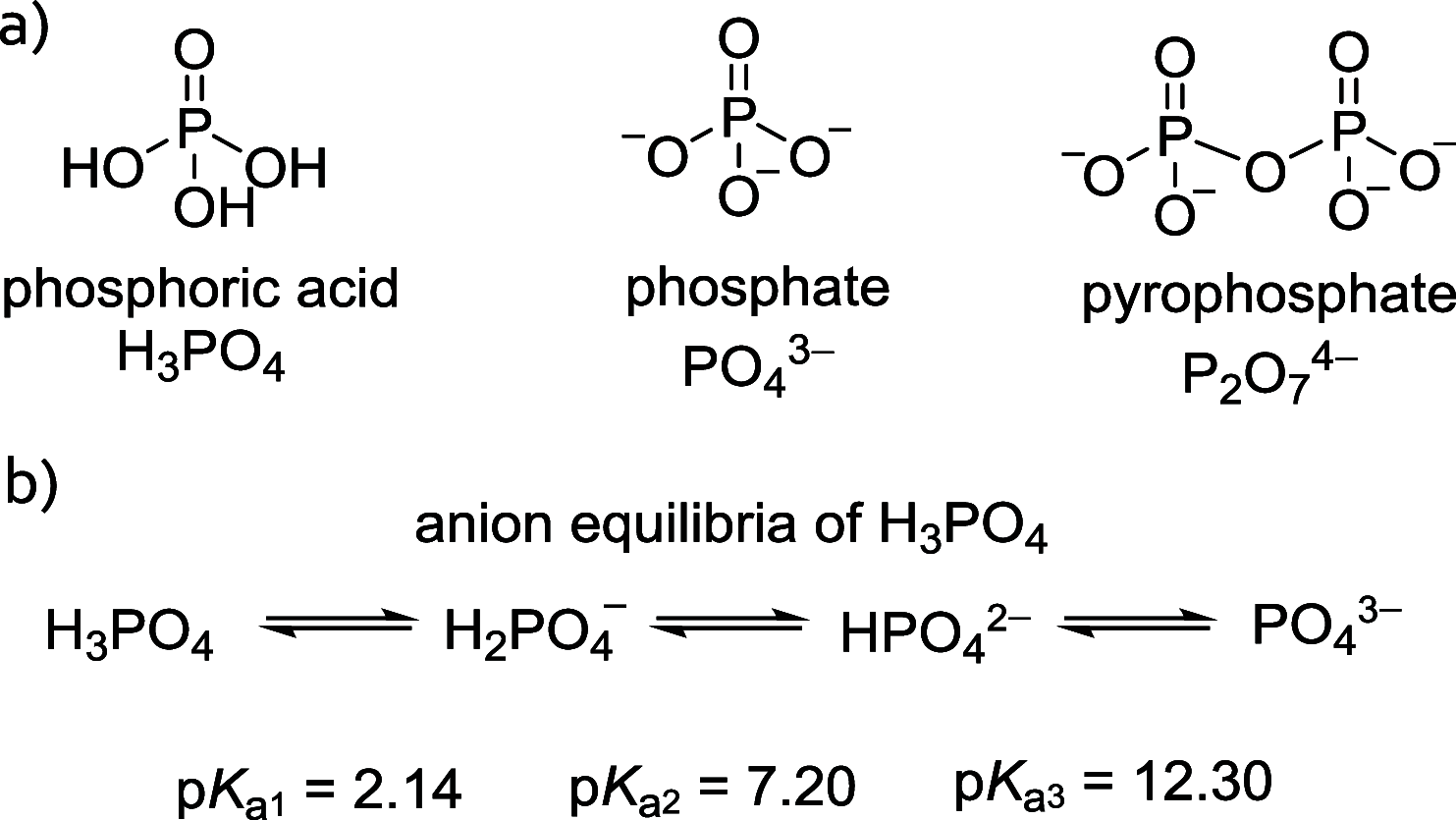
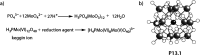
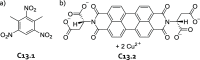
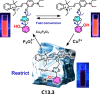
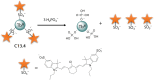

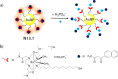
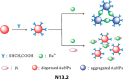


References
-
- Pedersen C. J. The Discovery of Crown Ethers (Noble Lecture). Angew. Chem., Int. Ed. Engl. 1988, 27, 1021–1027. 10.1002/anie.198810211. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

