Understanding the Adhesion Mechanism of Hydroxyapatite-Binding Peptide
- PMID: 34995466
- PMCID: PMC8793143
- DOI: 10.1021/acs.langmuir.1c02293
Understanding the Adhesion Mechanism of Hydroxyapatite-Binding Peptide
Abstract
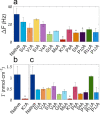
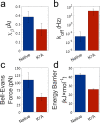
Understanding the interactions between the protein collagen and hydroxyapatite is of high importance for understanding biomineralization and bone formation. Here, we undertook a reductionist approach and studied the interactions between a short peptide and hydroxyapatite. The peptide was selected from a phage-display library for its high affinity to hydroxyapatite. To study its interactions with hydroxyapatite, we performed an alanine scan to determine the contribution of each residue. The interactions of the different peptide derivatives were studied using a quartz crystal microbalance with dissipation monitoring and with single-molecule force spectroscopy by atomic force microscopy. Our results suggest that the peptide binds via electrostatic interactions between cationic moieties of the peptide and the negatively charged groups on the crystal surface. Furthermore, our findings show that cationic residues have a crucial role in binding. Using molecular dynamics simulations, we show that the peptide structure is a contributing factor to the adhesion mechanism. These results suggest that even small conformational changes can have a significant effect on peptide adhesion. We suggest that a bent structure of the peptide allows it to strongly bind hydroxyapatite. The results presented in this study improve our understanding of peptide adhesion to hydroxyapatite. On top of physical interactions between the peptide and the surface, peptide structure contributes to adhesion. Unveiling these processes contributes to our understanding of more complex biological systems. Furthermore, it may help in the design of de novo peptides to be used as functional groups for modifying the surface of hydroxyapatite.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Olszta M. J.; Cheng X.; Jee S. S.; Kumar R.; Kim Y.-Y.; Kaufman M. J.; Douglas E. P.; Gower L. B. Bone structure and formation: A new perspective. Mater. Sci. Eng. R 2007, 58 (3–5), 77–116. 10.1016/j.mser.2007.05.001. - DOI
-
- Weiner S.; Wagner H. D. The material bone: structure-mechanical function relations. Annu. Rev. Mater. Sci. 1998, 28 (1), 271–298. 10.1146/annurev.matsci.28.1.271. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

