Central nervous system regeneration
- PMID: 34995518
- PMCID: PMC10896592
- DOI: 10.1016/j.cell.2021.10.029
Central nervous system regeneration
Abstract
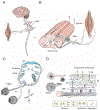
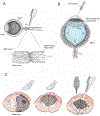
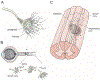
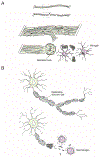
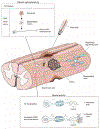
Neurons of the mammalian central nervous system fail to regenerate. Substantial progress has been made toward identifying the cellular and molecular mechanisms that underlie regenerative failure and how altering those pathways can promote cell survival and/or axon regeneration. Here, we summarize those findings while comparing the regenerative process in the central versus the peripheral nervous system. We also highlight studies that advance our understanding of the mechanisms underlying neural degeneration in response to injury, as many of these mechanisms represent primary targets for restoring functional neural circuits.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
References
-
- Ambros V, and Horvitz HR (1984). Heterochronic mutants of the nematode Caenorhabditis elegans. Science 226, 409–416. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

