Single-center experience using anakinra for steroid-refractory immune effector cell-associated neurotoxicity syndrome (ICANS)
- PMID: 34996813
- PMCID: PMC8744112
- DOI: 10.1136/jitc-2021-003847
Single-center experience using anakinra for steroid-refractory immune effector cell-associated neurotoxicity syndrome (ICANS)
Abstract
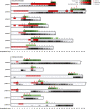
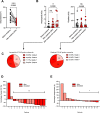
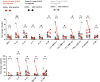
In addition to remarkable antitumor activity, chimeric antigen receptor (CAR) T-cell therapy is associated with acute toxicities such as cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS). Current treatment guidelines for CRS and ICANS include use of tocilizumab, a monoclonal antibody that blocks the interleukin (IL)-6 receptor, and corticosteroids. In patients with refractory CRS, use of several other agents as third-line therapy (including siltuximab, ruxolitinib, anakinra, dasatinib, and cyclophosphamide) has been reported on an anecdotal basis. At our institution, anakinra has become the standard treatment for the management of steroid-refractory ICANS with or without CRS, based on recent animal data demonstrating the role of IL-1 in the pathogenesis of ICANS/CRS. Here, we retrospectively analyzed clinical and laboratory parameters, including serum cytokines, in 14 patients at our center treated with anakinra for steroid-refractory ICANS with or without CRS after standard treatment with tisagenlecleucel (Kymriah) or axicabtagene ciloleucel (Yescarta) CD19-targeting CAR T. We observed statistically significant and rapid reductions in fever, inflammatory cytokines, and biomarkers associated with ICANS/CRS after anakinra treatment. With three daily subcutaneous doses, anakinra did not have a clear, clinically dramatic effect on neurotoxicity, and its use did not result in rapid tapering of corticosteroids; although neutropenia and thrombocytopenia were common at the time of anakinra dosing, there were no clear delays in hematopoietic recovery or infections that were directly attributable to anakinra. Anakinra may be useful adjunct to steroids and tocilizumab in the management of CRS and/or steroid-refractory ICANs resulting from CAR T-cell therapies, but prospective studies are needed to determine its efficacy in these settings.
Keywords: chimeric antigen receptors; combination; cytokines; drug therapy; inflammation.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: MW: patent: University of Bern Switzerland, CSL Behring, stockholder of Novartis and Nestle. KG, MBL, SLM, ARE-J, NH, POD, BD, DC, MT, KL: none. Y-BC: consulting for Incyte, Abbvie, Daiichi, Equilium, Actinium, and Celularity. ZDF: consulting for Syndax Pharmaceuticals Inc., Kadmon Corp., and Omeros Corp.; research support from Incyte Corp. and Regimmune Corp. TS: data safety monitoring board or advisory board of Bluebird Bio, Qihan Biotech, Jazz Pharmaceuticals, Syneos Health, and ITB-MED. MVM is an inventor of patents related to adoptive cell therapies, held by Massachusetts General Hospital and University of Pennsylvania (some licensed to Novartis) and holds equity in TCR2 and Century Therapeutics, serves on the Board of Directors of 2Seventy Bio, and has served as a consultant for multiple companies involved in cell therapies. MJF: consulting for Kite/Gilead, BMS, Novartis, Iovance, and Arcellx; research support from Kite/Gilead and Novartis.
Figures
Similar articles
-
CD19 CAR T-cell therapy and prophylactic anakinra in relapsed or refractory lymphoma: phase 2 trial interim results.Nat Med. 2023 Jul;29(7):1710-1717. doi: 10.1038/s41591-023-02404-6. Epub 2023 Jul 3. Nat Med. 2023. PMID: 37400640 Free PMC article. Clinical Trial.
-
Anakinra for Refractory Cytokine Release Syndrome or Immune Effector Cell-Associated Neurotoxicity Syndrome after Chimeric Antigen Receptor T Cell Therapy.Transplant Cell Ther. 2023 Jul;29(7):430-437. doi: 10.1016/j.jtct.2023.04.001. Epub 2023 Apr 7. Transplant Cell Ther. 2023. PMID: 37031746 Free PMC article.
-
Siltuximab for chimeric antigen receptor T-cell therapy-related CRS and ICANS: a multicenter retrospective analysis.Blood Adv. 2025 Jan 14;9(1):170-175. doi: 10.1182/bloodadvances.2024013688. Blood Adv. 2025. PMID: 39437770 Free PMC article.
-
Anticytokine Therapy and Corticosteroids for Cytokine Release Syndrome and for Neurotoxicity Following T-Cell Engager or CAR T-Cell Therapy: Rapid Review [Internet].Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2024 May. Report No.: RC1534. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2024 May. Report No.: RC1534. PMID: 38985918 Free Books & Documents. Review.
-
Cytokine release syndrome and neurotoxicity following CAR T-cell therapy for hematologic malignancies.J Allergy Clin Immunol. 2020 Nov;146(5):940-948. doi: 10.1016/j.jaci.2020.07.025. Epub 2020 Aug 6. J Allergy Clin Immunol. 2020. PMID: 32771558 Review.
Cited by
-
Overview of infectious complications among CAR T- cell therapy recipients.Front Oncol. 2024 Jul 3;14:1398078. doi: 10.3389/fonc.2024.1398078. eCollection 2024. Front Oncol. 2024. PMID: 39026972 Free PMC article. Review.
-
Application and Design of Switches Used in CAR.Cells. 2022 Jun 13;11(12):1910. doi: 10.3390/cells11121910. Cells. 2022. PMID: 35741039 Free PMC article. Review.
-
Exploring CAR-T Cell Therapy Side Effects: Mechanisms and Management Strategies.J Clin Med. 2023 Sep 22;12(19):6124. doi: 10.3390/jcm12196124. J Clin Med. 2023. PMID: 37834768 Free PMC article. Review.
-
CAR-T in the Treatment of Acute Myeloid Leukemia: Barriers and How to Overcome Them.Hemasphere. 2023 Aug 18;7(9):e937. doi: 10.1097/HS9.0000000000000937. eCollection 2023 Sep. Hemasphere. 2023. PMID: 37674860 Free PMC article. Review.
-
CD19 CAR T-cell therapy and prophylactic anakinra in relapsed or refractory lymphoma: phase 2 trial interim results.Nat Med. 2023 Jul;29(7):1710-1717. doi: 10.1038/s41591-023-02404-6. Epub 2023 Jul 3. Nat Med. 2023. PMID: 37400640 Free PMC article. Clinical Trial.
References
-
- Topp MS, van Meerten T, Houot R, et al. . Earlier steroid use with Axicabtagene Ciloleucel (Axi-Cel) in patients with relapsed/refractory large B cell lymphoma (R/R LBCL). Biology of Blood and Marrow Transplantation 2020;26:S101. 10.1016/j.bbmt.2019.12.603 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
