Imaging active site chemistry and protonation states: NMR crystallography of the tryptophan synthase α-aminoacrylate intermediate
- PMID: 34996869
- PMCID: PMC8764694
- DOI: 10.1073/pnas.2109235119
Imaging active site chemistry and protonation states: NMR crystallography of the tryptophan synthase α-aminoacrylate intermediate
Abstract
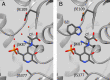
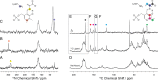
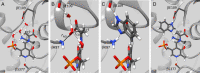
NMR-assisted crystallography-the integrated application of solid-state NMR, X-ray crystallography, and first-principles computational chemistry-holds significant promise for mechanistic enzymology: by providing atomic-resolution characterization of stable intermediates in enzyme active sites, including hydrogen atom locations and tautomeric equilibria, NMR crystallography offers insight into both structure and chemical dynamics. Here, this integrated approach is used to characterize the tryptophan synthase α-aminoacrylate intermediate, a defining species for pyridoxal-5'-phosphate-dependent enzymes that catalyze β-elimination and replacement reactions. For this intermediate, NMR-assisted crystallography is able to identify the protonation states of the ionizable sites on the cofactor, substrate, and catalytic side chains as well as the location and orientation of crystallographic waters within the active site. Most notable is the water molecule immediately adjacent to the substrate β-carbon, which serves as a hydrogen bond donor to the ε-amino group of the acid-base catalytic residue βLys87. From this analysis, a detailed three-dimensional picture of structure and reactivity emerges, highlighting the fate of the L-serine hydroxyl leaving group and the reaction pathway back to the preceding transition state. Reaction of the α-aminoacrylate intermediate with benzimidazole, an isostere of the natural substrate indole, shows benzimidazole bound in the active site and poised for, but unable to initiate, the subsequent bond formation step. When modeled into the benzimidazole position, indole is positioned with C3 in contact with the α-aminoacrylate Cβ and aligned for nucleophilic attack. Here, the chemically detailed, three-dimensional structure from NMR-assisted crystallography is key to understanding why benzimidazole does not react, while indole does.
Keywords: NMR-assisted crystallography; integrated structural biology; pyridoxal-5′-phosphate; solid-state NMR; tryptophan synthase.
Copyright © 2022 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures




Similar articles
-
NMR crystallography of enzyme active sites: probing chemically detailed, three-dimensional structure in tryptophan synthase.Acc Chem Res. 2013 Sep 17;46(9):2008-17. doi: 10.1021/ar3003333. Epub 2013 Mar 28. Acc Chem Res. 2013. PMID: 23537227 Free PMC article.
-
X-ray and NMR crystallography in an enzyme active site: the indoline quinonoid intermediate in tryptophan synthase.J Am Chem Soc. 2011 Jan 12;133(1):4-7. doi: 10.1021/ja106555c. Epub 2010 Dec 10. J Am Chem Soc. 2011. PMID: 21142052
-
NMR Crystallography of a Carbanionic Intermediate in Tryptophan Synthase: Chemical Structure, Tautomerization, and Reaction Specificity.J Am Chem Soc. 2016 Nov 23;138(46):15214-15226. doi: 10.1021/jacs.6b08937. Epub 2016 Nov 11. J Am Chem Soc. 2016. PMID: 27779384 Free PMC article.
-
Stereospecificity of alpha-proton exchange reactions catalysed by pyridoxal-5'-phosphate-dependent enzymes.Biochim Biophys Acta. 2003 Apr 11;1647(1-2):138-42. doi: 10.1016/s1570-9639(03)00080-3. Biochim Biophys Acta. 2003. PMID: 12686123 Review.
-
Tryptophan synthase: a multienzyme complex with an intramolecular tunnel.Chem Rec. 2001;1(2):140-51. doi: 10.1002/tcr.4. Chem Rec. 2001. PMID: 11893063 Review.
Cited by
-
Uniform chi-squared model probabilities in NMR crystallography.Faraday Discuss. 2025 Jan 8;255(0):203-221. doi: 10.1039/d4fd00114a. Faraday Discuss. 2025. PMID: 39311003
-
Neutron diffraction from a microgravity-grown crystal reveals the active site hydrogens of the internal aldimine form of tryptophan synthase.Cell Rep Phys Sci. 2024 Feb 21;5(2):101827. doi: 10.1016/j.xcrp.2024.101827. Epub 2024 Feb 12. Cell Rep Phys Sci. 2024. PMID: 38645802 Free PMC article.
-
Spiers Memorial Lecture: NMR crystallography.Faraday Discuss. 2025 Jan 8;255(0):9-45. doi: 10.1039/d4fd00151f. Faraday Discuss. 2025. PMID: 39405130 Free PMC article.
-
Crystal structure validation of verinurad via proton-detected ultra-fast MAS NMR and machine learning.Faraday Discuss. 2025 Jan 8;255(0):143-158. doi: 10.1039/d4fd00076e. Faraday Discuss. 2025. PMID: 39297322 Free PMC article.
-
Solid-state NMR MAS CryoProbe enables structural studies of human blood protein vitronectin bound to hydroxyapatite.J Struct Biol. 2024 Mar;216(1):108061. doi: 10.1016/j.jsb.2024.108061. Epub 2024 Jan 5. J Struct Biol. 2024. PMID: 38185342 Free PMC article.
References
-
- Walsh C., Enzymatic Reaction Mechanisms (W. H. Freeman and Company, San Francisco, 1979), pp. 978.
-
- Hayashi H., Pyridoxal enzymes: Mechanistic diversity and uniformity. J. Biochem. 118, 463–473 (1995). - PubMed
-
- Miles E. W., Tryptophan synthase: Structure, function, and subunit interaction. Adv. Enzymol. Relat. Areas Mol. Biol. 49, 127–186 (1979). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

