FASN-dependent de novo lipogenesis is required for brain development
- PMID: 34996870
- PMCID: PMC8764667
- DOI: 10.1073/pnas.2112040119
FASN-dependent de novo lipogenesis is required for brain development
Abstract
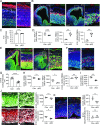
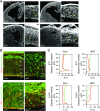
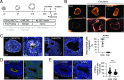
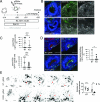
Fate and behavior of neural progenitor cells are tightly regulated during mammalian brain development. Metabolic pathways, such as glycolysis and oxidative phosphorylation, that are required for supplying energy and providing molecular building blocks to generate cells govern progenitor function. However, the role of de novo lipogenesis, which is the conversion of glucose into fatty acids through the multienzyme protein fatty acid synthase (FASN), for brain development remains unknown. Using Emx1Cre-mediated, tissue-specific deletion of Fasn in the mouse embryonic telencephalon, we show that loss of FASN causes severe microcephaly, largely due to altered polarity of apical, radial glia progenitors and reduced progenitor proliferation. Furthermore, genetic deletion and pharmacological inhibition of FASN in human embryonic stem cell-derived forebrain organoids identifies a conserved role of FASN-dependent lipogenesis for radial glia cell polarity in human brain organoids. Thus, our data establish a role of de novo lipogenesis for mouse and human brain development and identify a link between progenitor-cell polarity and lipid metabolism.
Keywords: lipogenesis; neural stem cell; neurogenesis; polarity.
Copyright © 2022 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures
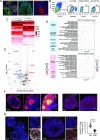
Similar articles
-
Metabolic control of adult neural stem cell activity by Fasn-dependent lipogenesis.Nature. 2013 Jan 10;493(7431):226-30. doi: 10.1038/nature11689. Epub 2012 Dec 2. Nature. 2013. PMID: 23201681 Free PMC article.
-
Mitogenic Sonic hedgehog signaling drives E2F1-dependent lipogenesis in progenitor cells and medulloblastoma.Oncogene. 2011 Jan 27;30(4):410-22. doi: 10.1038/onc.2010.454. Epub 2010 Oct 4. Oncogene. 2011. PMID: 20890301 Free PMC article.
-
Differential requirement for de novo lipogenesis in cholangiocarcinoma and hepatocellular carcinoma of mice and humans.Hepatology. 2016 Jun;63(6):1900-13. doi: 10.1002/hep.28508. Epub 2016 Mar 25. Hepatology. 2016. PMID: 26910791 Free PMC article.
-
The mitochondrial H(+)-ATP synthase and the lipogenic switch: new core components of metabolic reprogramming in induced pluripotent stem (iPS) cells.Cell Cycle. 2013 Jan 15;12(2):207-18. doi: 10.4161/cc.23352. Epub 2012 Jan 15. Cell Cycle. 2013. PMID: 23287468 Free PMC article. Review.
-
The implications of FASN in immune cell biology and related diseases.Cell Death Dis. 2024 Jan 25;15(1):88. doi: 10.1038/s41419-024-06463-6. Cell Death Dis. 2024. PMID: 38272906 Free PMC article. Review.
Cited by
-
Fatty acid synthase global inducible knockout does not alter brain fatty acid concentrations but attenuates cholesterol synthesis in the adult mouse.Prostaglandins Leukot Essent Fatty Acids. 2025 Jul;205:102679. doi: 10.1016/j.plefa.2025.102679. Epub 2025 Mar 31. Prostaglandins Leukot Essent Fatty Acids. 2025. PMID: 40185011
-
Injection and electroporation of plasmid DNA into human cortical organoids.STAR Protoc. 2022 Jan 20;3(1):101129. doi: 10.1016/j.xpro.2022.101129. eCollection 2022 Mar 18. STAR Protoc. 2022. PMID: 35118433 Free PMC article.
-
Epigenetic regulation of H3K27me3 in laying hens with fatty liver hemorrhagic syndrome induced by high-energy and low-protein diets.BMC Genomics. 2024 Apr 16;25(1):374. doi: 10.1186/s12864-024-10270-w. BMC Genomics. 2024. PMID: 38627644 Free PMC article.
-
Brain energy metabolism: A roadmap for future research.J Neurochem. 2024 May;168(5):910-954. doi: 10.1111/jnc.16032. Epub 2024 Jan 6. J Neurochem. 2024. PMID: 38183680 Free PMC article. Review.
-
A fluorescent perilipin 2 knock-in mouse model reveals a high abundance of lipid droplets in the developing and adult brain.Nat Commun. 2024 Jun 28;15(1):5489. doi: 10.1038/s41467-024-49449-w. Nat Commun. 2024. PMID: 38942786 Free PMC article.
References
-
- Taverna E., Götz M., Huttner W. B., The cell biology of neurogenesis: Toward an understanding of the development and evolution of the neocortex. Annu. Rev. Cell Dev. Biol. 30, 465–502 (2014). - PubMed
-
- Oberst P., Agirman G., Jabaudon D., Principles of progenitor temporal patterning in the developing invertebrate and vertebrate nervous system. Curr. Opin. Neurobiol. 56, 185–193 (2019). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

