Structural transitions in the GTP cap visualized by cryo-electron microscopy of catalytically inactive microtubules
- PMID: 34996871
- PMCID: PMC8764682
- DOI: 10.1073/pnas.2114994119
Structural transitions in the GTP cap visualized by cryo-electron microscopy of catalytically inactive microtubules
Abstract
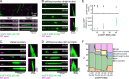
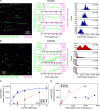
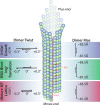
Microtubules (MTs) are polymers of αβ-tubulin heterodimers that stochastically switch between growth and shrinkage phases. This dynamic instability is critically important for MT function. It is believed that GTP hydrolysis within the MT lattice is accompanied by destabilizing conformational changes and that MT stability depends on a transiently existing GTP cap at the growing MT end. Here, we use cryo-electron microscopy and total internal reflection fluorescence microscopy of GTP hydrolysis-deficient MTs assembled from mutant recombinant human tubulin to investigate the structure of a GTP-bound MT lattice. We find that the GTP-MT lattice of two mutants in which the catalytically active glutamate in α-tubulin was substituted by inactive amino acids (E254A and E254N) is remarkably plastic. Undecorated E254A and E254N MTs with 13 protofilaments both have an expanded lattice but display opposite protofilament twists, making these lattices distinct from the compacted lattice of wild-type GDP-MTs. End-binding proteins of the EB family have the ability to compact both mutant GTP lattices and to stabilize a negative twist, suggesting that they promote this transition also in the GTP cap of wild-type MTs, thereby contributing to the maturation of the MT structure. We also find that the MT seam appears to be stabilized in mutant GTP-MTs and destabilized in GDP-MTs, supporting the proposal that the seam plays an important role in MT stability. Together, these structures of catalytically inactive MTs add mechanistic insight into the GTP state of MTs, the stability of the GTP- and GDP-bound lattice, and our overall understanding of MT dynamic instability.
Keywords: GTP; TIRF microscopy; cryo-EM; dynamic instability; microtubules.
Copyright © 2022 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures



Similar articles
-
Hydrolysis-deficient mosaic microtubules as faithful mimics of the GTP cap.Nat Commun. 2025 Mar 10;16(1):2396. doi: 10.1038/s41467-025-57555-6. Nat Commun. 2025. PMID: 40064882 Free PMC article.
-
The structure of microtubule ends during the elongation and shortening phases of dynamic instability examined by negative-stain electron microscopy.J Cell Sci. 1990 Aug;96 ( Pt 4):571-82. doi: 10.1242/jcs.96.4.571. J Cell Sci. 1990. PMID: 2283357
-
Mechanistic Origin of Microtubule Dynamic Instability and Its Modulation by EB Proteins.Cell. 2015 Aug 13;162(4):849-59. doi: 10.1016/j.cell.2015.07.012. Epub 2015 Jul 30. Cell. 2015. PMID: 26234155 Free PMC article.
-
Visualizing microtubule structural transitions and interactions with associated proteins.Curr Opin Struct Biol. 2016 Apr;37:90-6. doi: 10.1016/j.sbi.2015.12.009. Epub 2016 Jan 21. Curr Opin Struct Biol. 2016. PMID: 26803284 Free PMC article. Review.
-
Molecular mechanisms underlying microtubule growth dynamics.Curr Biol. 2021 May 24;31(10):R560-R573. doi: 10.1016/j.cub.2021.02.035. Curr Biol. 2021. PMID: 34033790 Free PMC article. Review.
Cited by
-
Protein folding: Funnel model revised.Comput Struct Biotechnol J. 2024 Oct 21;23:3827-3838. doi: 10.1016/j.csbj.2024.10.030. eCollection 2024 Dec. Comput Struct Biotechnol J. 2024. PMID: 39525086 Free PMC article.
-
Hydrolysis-deficient mosaic microtubules as faithful mimics of the GTP cap.Nat Commun. 2025 Mar 10;16(1):2396. doi: 10.1038/s41467-025-57555-6. Nat Commun. 2025. PMID: 40064882 Free PMC article.
-
Mechanism and regulation of kinesin motors.Nat Rev Mol Cell Biol. 2025 Feb;26(2):86-103. doi: 10.1038/s41580-024-00780-6. Epub 2024 Oct 11. Nat Rev Mol Cell Biol. 2025. PMID: 39394463 Review.
-
Purification, Fluorescent Labeling, and Detyrosination of Mammalian Cell Tubulin for Biochemical Assays.Cytoskeleton (Hoboken). 2025 Jul 12:10.1002/cm.70005. doi: 10.1002/cm.70005. Online ahead of print. Cytoskeleton (Hoboken). 2025. PMID: 40650557
-
Unveiling the catalytic mechanism of GTP hydrolysis in microtubules.Proc Natl Acad Sci U S A. 2023 Jul 4;120(27):e2305899120. doi: 10.1073/pnas.2305899120. Epub 2023 Jun 26. Proc Natl Acad Sci U S A. 2023. PMID: 37364095 Free PMC article.
References
-
- Drechsel D. N., Kirschner M. W., The minimum GTP cap required to stabilize microtubules. Curr. Biol. 4, 1053–1061 (1994). - PubMed
-
- Howard J., Hyman A. A., Growth, fluctuation and switching at microtubule plus ends. Nat. Rev. Mol. Cell Biol. 10, 569–574 (2009). - PubMed
-
- Mitchison T., Kirschner M., Dynamic instability of microtubule growth. Nature 312, 237–242 (1984). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

