High BRCA1 gene expression increases the risk of early distant metastasis in ER+ breast cancers
- PMID: 34996912
- PMCID: PMC8741892
- DOI: 10.1038/s41598-021-03471-w
High BRCA1 gene expression increases the risk of early distant metastasis in ER+ breast cancers
Abstract
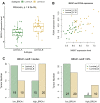
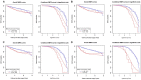
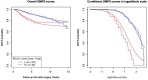
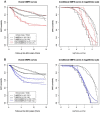
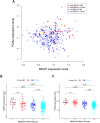
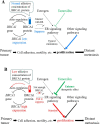
Although the function of the BRCA1 gene has been extensively studied, the relationship between BRCA1 gene expression and tumor aggressiveness remains controversial in sporadic breast cancers. Because the BRCA1 protein is known to regulate estrogen signaling, we selected microarray data of ER+ breast cancers from the GEO public repository to resolve previous conflicting findings. The BRCA1 gene expression level in highly proliferative luminal B tumors was shown to be higher than that in luminal A tumors. Survival analysis using a cure model indicated that patients of early ER+ breast cancers with high BRCA1 expression developed rapid distant metastasis. In addition, the proliferation marker genes MKI67 and PCNA, which are characteristic of aggressive tumors, were also highly expressed in patients with high BRCA1 expression. The associations among high BRCA1 expression, high proliferation marker expression, and high risk of distant metastasis emerged in independent datasets, regardless of tamoxifen treatment. Tamoxifen therapy could improve the metastasis-free fraction of high BRCA1 expression patients. Our findings link BRCA1 expression with proliferation and possibly distant metastasis via the ER signaling pathway. We propose a testable hypothesis based on these consistent results and offer an interpretation for our reported associations.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Mangia A, et al. BRCA1 expression and molecular alterations in familial breast cancer. Histol. Histopathol. 2009;24:69–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

