Ablation of VLA4 in multiple myeloma cells redirects tumor spread and prolongs survival
- PMID: 34996933
- PMCID: PMC8741970
- DOI: 10.1038/s41598-021-03748-0
Ablation of VLA4 in multiple myeloma cells redirects tumor spread and prolongs survival
Abstract
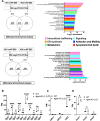
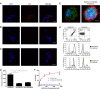
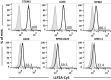
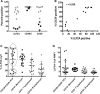
Multiple myeloma (MM) is a cancer of bone marrow (BM) plasma cells, which is increasingly treatable but still incurable. In 90% of MM patients, severe osteolysis results from pathological interactions between MM cells and the bone microenvironment. Delineating specific molecules and pathways for their role in cancer supportive interactions in the BM is vital for developing new therapies. Very Late Antigen 4 (VLA4, integrin α4β1) is a key player in cell-cell adhesion and signaling between MM and BM cells. We evaluated a VLA4 selective near infrared fluorescent probe, LLP2A-Cy5, for in vitro and in vivo optical imaging of VLA4. Furthermore, two VLA4-null murine 5TGM1 MM cell (KO) clones were generated by CRISPR/Cas9 knockout of the Itga4 (α4) subunit, which induced significant alterations in the transcriptome. In contrast to the VLA4+ 5TGM1 parental cells, C57Bl/KaLwRij immunocompetent syngeneic mice inoculated with the VLA4-null clones showed prolonged survival, reduced medullary disease, and increased extramedullary disease burden. The KO tumor foci showed significantly reduced uptake of LLP2A-Cy5, confirming in vivo specificity of this imaging agent. This work provides new insights into the pathogenic role of VLA4 in MM, and evaluates an optical tool to measure its expression in preclinical models.
© 2022. The Author(s).
Conflict of interest statement
JFD receives research support from Macrogenics, NeoImmuneTech and Bioline; is a consultant of REvervest; received honorarium Amgen and has ownership/equity in Magenta, WUGEN. MS is the co-founder of Sarya, LLC. All other authors declare no competing financial interests.
Figures


References
-
- Howlader, N.N.A., Krapcho, M., Miller, D., Brest, A., Yu, M., Ruhl, J., Tatalovich, Z., Mariotto, A., Lewis, D.R., Chen, H.S., Feuer, E.J., Cronin, K.A. (eds). SEER Cancer Statistics Review, 1975–2018, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2018/, based on November 2020 SEER data submission, posted to the SEER web site, April 2021.
-
- Mina R, Joseph NS, Gay F, Kastritis E, Petrucci MT, Kaufman JL, Montefusco V, Gavriatopoulou M, Patriarca F, Omedé P, Boise LH, Roussou M, Giuliani N, Oliva S, Offidani M, Belotti A, Jaye DL, De Paoli L, Terpos E, Lonial S, Boccadoro M, Nooka AK, Dimopoulos MA. Clinical features and survival of multiple myeloma patients harboring t(14;16) in the era of novel agents. Blood Cancer J. 2020;10:40. - PMC - PubMed
-
- Lentzsch S, Ehrlich LA, Roodman GD. Pathophysiology of multiple myeloma bone disease. Hematol. Oncol. Clin. North Am. 2007;21:1035–1049. - PubMed
-
- Terpos, E., Christoulas, D., Gavriatopoulou, M. and Dimopoulos, M.A. Mechanisms of bone destruction in multiple myeloma. Eur. J. Cancer Care (Engl). 2017;26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 CA094056/CA/NCI NIH HHS/United States
- T32 EB014855/EB/NIBIB NIH HHS/United States
- R01 CA248493/NH/NIH HHS/United States
- R50 CA211466/CA/NCI NIH HHS/United States
- NIH -S10OD027042/NH/NIH HHS/United States
- U54 CA199092/CA/NCI NIH HHS/United States
- S10 OD027042/OD/NIH HHS/United States
- P30 CA091842/CA/NCI NIH HHS/United States
- R01 CA176221/CA/NCI NIH HHS/United States
- P30 DK056341/DK/NIDDK NIH HHS/United States
- R01 CA248493/CA/NCI NIH HHS/United States
- R42 CA257797/CA/NCI NIH HHS/United States
- U54 CA199092/NH/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials

