Baseline predictors for subretinal fibrosis in neovascular age-related macular degeneration
- PMID: 34996934
- PMCID: PMC8741927
- DOI: 10.1038/s41598-021-03716-8
Baseline predictors for subretinal fibrosis in neovascular age-related macular degeneration
Abstract
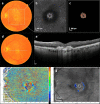
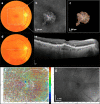
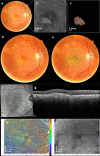
To find baseline predictors for subretinal fibrosis (SF) in neovascular age-related macular degeneration (nAMD). Forty-five eyes of 45 participants with treatment-naïve nAMD were consecutively enrolled and treated according to a standardized treat-and-extend protocol. Spectral-domain optical coherence tomography (OCT), color fundus photography and fluorescein angiography as well as novel imaging modalities polarization-sensitive OCT and OCT angiography (OCTA) were performed to detect SF after 1 year and find baseline predictors for SF development. Baseline OCTA scans were evaluated for quantitative features such as lesion area, vessel area, vessel junctions, vessel length, vessel endpoints and mean lacunarity. Additionally, the type of macular neovascularization, the presence of subretinal fluid, intraretinal fluid (IRF), subretinal hyperreflective material (SHRM), retinal hemorrhage as well as best-corrected visual acuity (BCVA) were evaluated. After 12 months 8 eyes (18%) developed SF. Eyes with SF had worse baseline BCVA (p = .001) and a higher prevalence of IRF (p = .014) and SHRM at baseline (p = .017). There was no significant difference in any of the evaluated quantitative OCTA parameters (p > .05) between eyes with and without SF. There were no quantitative baseline microvascular predictors for SF in our study. Low baseline BCVA, the presence of IRF and SHRM, however, are easily identifiable baseline parameters indicating increased risk.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Analysis of factors affecting prognosis of the visual acuity and baseline risk factors for subretinal fibrosis in neovascular age-related macular degeneration patients.Front Med (Lausanne). 2024 Nov 28;11:1451726. doi: 10.3389/fmed.2024.1451726. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39669991 Free PMC article.
-
Variable response of subretinal hyperreflective material to anti-vascular endothelial growth factor classified with optical coherence tomography angiography.Graefes Arch Clin Exp Ophthalmol. 2018 Nov;256(11):2089-2096. doi: 10.1007/s00417-018-4121-7. Epub 2018 Sep 1. Graefes Arch Clin Exp Ophthalmol. 2018. PMID: 30173338
-
PROGNOSTIC VALUE OF SUBRETINAL HYPERREFLECTIVE MATERIAL IN NEOVASCULAR AGE-RELATED MACULAR DEGENERATION TREATED WITH BEVACIZUMAB.Retina. 2018 Aug;38(8):1485-1491. doi: 10.1097/IAE.0000000000001748. Retina. 2018. PMID: 28654630
-
Subretinal fibrosis occurrence according to macular neovascularisation subtypes in neovascular age-related macular degeneration.Acta Ophthalmol. 2025 Mar;103(2):e104-e117. doi: 10.1111/aos.16759. Epub 2024 Sep 25. Acta Ophthalmol. 2025. PMID: 39319643
-
Association Between Visual Acuity and Residual Retinal Fluid Following Intravitreal Anti-Vascular Endothelial Growth Factor Treatment for Neovascular Age-Related Macular Degeneration: A Systematic Review and Meta-analysis.JAMA Ophthalmol. 2022 Jun 1;140(6):611-622. doi: 10.1001/jamaophthalmol.2022.1357. JAMA Ophthalmol. 2022. PMID: 35551359 Free PMC article.
Cited by
-
Different Therapeutic Approaches for Dry and Wet AMD.Int J Mol Sci. 2024 Dec 4;25(23):13053. doi: 10.3390/ijms252313053. Int J Mol Sci. 2024. PMID: 39684764 Free PMC article. Review.
-
Diagnostic Accuracy of the Amsler Grid Test for Detecting Neovascular Age-Related Macular Degeneration: A Systematic Review and Meta-analysis.JAMA Ophthalmol. 2023 Apr 1;141(4):315-323. doi: 10.1001/jamaophthalmol.2022.6396. JAMA Ophthalmol. 2023. PMID: 36795396 Free PMC article.
-
Associations of presenting visual acuity with morphological changes on OCT in neovascular age-related macular degeneration: PRECISE Study Report 2.Eye (Lond). 2024 Mar;38(4):757-765. doi: 10.1038/s41433-023-02769-5. Epub 2023 Oct 18. Eye (Lond). 2024. PMID: 37853106 Free PMC article.
-
Automated fluid monitoring to optimize the follow-up of neovascular age-related macular degeneration patients in the Brazilian population.Int J Retina Vitreous. 2025 Jul 6;11(1):75. doi: 10.1186/s40942-025-00695-0. Int J Retina Vitreous. 2025. PMID: 40619442 Free PMC article.
-
15 years of anti-VEGF treatment for nAMD: success or failure or something in between?Eye (Lond). 2022 Dec;36(12):2232-2233. doi: 10.1038/s41433-022-02153-9. Epub 2022 Jun 25. Eye (Lond). 2022. PMID: 35752715 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

