Soluble programmed cell death-1 predicts hepatocellular carcinoma development during nucleoside analogue treatment
- PMID: 34996935
- PMCID: PMC8741806
- DOI: 10.1038/s41598-021-03706-w
Soluble programmed cell death-1 predicts hepatocellular carcinoma development during nucleoside analogue treatment
Abstract
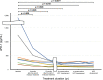
Soluble immune checkpoint molecules are emerging novel mediators of immune regulation. However, it is unclear whether soluble immune checkpoint proteins affect the development of hepatocellular carcinoma (HCC) during nucleos(t)ide analogue (NA) treatment in patients with chronic hepatitis B virus infection. This study included 122 NA-naïve patients who received NA therapy. We assessed the associations of clinical factors, including soluble immune checkpoint proteins, with HCC development during NA treatment. The baseline serum concentrations of 16 soluble immune checkpoint proteins were measured using multiplexed fluorescent bead-based immunoassay. In total, 13 patients developed HCC during the follow-up period (median duration, 4.3 years). Of the 16 proteins, soluble inducible T-cell co-stimulator (≥ 164.71 pg/mL; p = 0.014), soluble programmed cell death-1 (sPD-1) (≤ 447.27 pg/mL; p = 0.031), soluble CD40 (≤ 493.68 pg/mL; p = 0.032), and soluble herpes virus entry mediator (≤ 2470.83 pg/mL; p = 0.038) were significantly associated with HCC development (log-rank test). In multivariate analysis, an sPD-1 level ≤ 447.27 pg/mL (p = 0.014; hazard ratio [HR], 4.537) and α-fetoprotein level ≥ 6.4 ng/mL (p = 0.040; HR, 5.524) were independently and significantly associated with HCC development. Pre-treatment sPD-1 is a novel predictive biomarker for HCC development during NA treatment.
© 2022. The Author(s).
Conflict of interest statement
In the past year, Dr. Sawako Uchida-Kobayashi has received research funding from Bristol-Myers. The other authors declare that they have no competing interests.
Figures



References
-
- European Association for the Study of the Liver EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J. Hepatol. 2017;67:370–398. - PubMed
-
- Liaw YF, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N. Engl. J. Med. 2004;351:1521–1531. - PubMed
-
- Hiramatsu N, Yamada R, Takehara T. The suppressive effect of nucleos(t)ide analogue treatment on the incidence of hepatocellular carcinoma in chronic hepatitis B patients. J. Gastroenterol. Hepatol. 2016;31:546–552. - PubMed
-
- Hosaka T, et al. Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology. 2013;58:98–107. - PubMed
-
- Yamada R, et al. Impact of alpha-fetoprotein on hepatocellular carcinoma development during entecavir treatment of chronic hepatitis B virus infection. J. Gastroenterol. 2015;50:785–794. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

