Decrease of Pdzrn3 is required for heart maturation and protects against heart failure
- PMID: 34996942
- PMCID: PMC8742099
- DOI: 10.1038/s41598-021-03795-7
Decrease of Pdzrn3 is required for heart maturation and protects against heart failure
Abstract
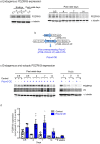
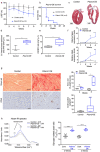
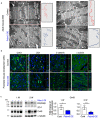
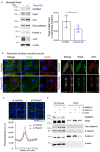
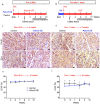
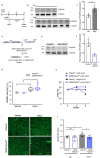
Heart failure is the final common stage of most cardiopathies. Cardiomyocytes (CM) connect with others via their extremities by intercalated disk protein complexes. This planar and directional organization of myocytes is crucial for mechanical coupling and anisotropic conduction of the electric signal in the heart. One of the hallmarks of heart failure is alterations in the contact sites between CM. Yet no factor on its own is known to coordinate CM polarized organization. We have previously shown that PDZRN3, an ubiquitine ligase E3 expressed in various tissues including the heart, mediates a branch of the Planar cell polarity (PCP) signaling involved in tissue patterning, instructing cell polarity and cell polar organization within a tissue. PDZRN3 is expressed in the embryonic mouse heart then its expression dropped significantly postnatally corresponding with heart maturation and CM polarized elongation. A moderate CM overexpression of Pdzrn3 (Pdzrn3 OE) during the first week of life, induced a severe eccentric hypertrophic phenotype with heart failure. In models of pressure-overload stress heart failure, CM-specific Pdzrn3 knockout showed complete protection against degradation of heart function. We reported that Pdzrn3 signaling induced PKC ζ expression, c-Jun nuclear translocation and a reduced nuclear ß catenin level, consistent markers of the planar non-canonical Wnt signaling in CM. We then show that subcellular localization (intercalated disk) of junction proteins as Cx43, ZO1 and Desmoglein 2 was altered in Pdzrn3 OE mice, which provides a molecular explanation for impaired CM polarization in these mice. Our results reveal a novel signaling pathway that controls a genetic program essential for heart maturation and maintenance of overall geometry, as well as the contractile function of CM, and implicates PDZRN3 as a potential therapeutic target for the prevention of human heart failure.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Balse E, Steele DF, Abriel H, Coulombe A, Fedida D, Hatem SN. Dynamic of ion channel expression at the plasma membrane of cardiomyocytes. Physiol. Rev. 2012;92:1317–1358. - PubMed
-
- Le Garrec J-F, Ragni CV, Pop S, Dufour A, Olivo-Marin J-C, Buckingham ME, Meilhac SM. Quantitative analysis of polarity in 3D reveals local cell coordination in the embryonic mouse heart. Development. 2013;140:395–404. - PubMed
-
- Pandur P, Lasche M, Eisenberg LM, Kuhl M. Wnt-11 activation of a non-canonical Wnt signalling pathway is required for cardiogenesis. Nature. 2002;418:636–641. - PubMed
-
- Nagy I, Railo A, Rapila R, Hast T, Sormunen R, Tavi P, Rasanen J, Vainio SJ. Wnt-11 signalling controls ventricular myocardium development by patterning N-cadherin and beta-catenin expression. Cardiovasc. Res. 2010;85:100–109. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

