Single-cell transcriptomics defines keratinocyte differentiation in avian scutate scales
- PMID: 34997067
- PMCID: PMC8742010
- DOI: 10.1038/s41598-021-04082-1
Single-cell transcriptomics defines keratinocyte differentiation in avian scutate scales
Abstract
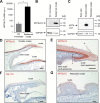
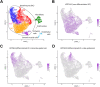
The growth of skin appendages, such as hair, feathers and scales, depends on terminal differentiation of epidermal keratinocytes. Here, we investigated keratinocyte differentiation in avian scutate scales. Cells were isolated from the skin on the legs of 1-day old chicks and subjected to single-cell transcriptomics. We identified two distinct populations of differentiated keratinocytes. The first population was characterized by mRNAs encoding cysteine-rich keratins and corneous beta-proteins (CBPs), also known as beta-keratins, of the scale type, indicating that these cells form hard scales. The second population of differentiated keratinocytes contained mRNAs encoding cysteine-poor keratins and keratinocyte-type CBPs, suggesting that these cells form the soft interscale epidermis. We raised an antibody against keratin 9-like cysteine-rich 2 (KRT9LC2), which is encoded by an mRNA enriched in the first keratinocyte population. Immunostaining confirmed expression of KRT9LC2 in the suprabasal epidermal layers of scutate scales but not in interscale epidermis. Keratinocyte differentiation in chicken leg skin resembled that in human skin with regard to the transcriptional upregulation of epidermal differentiation complex genes and genes involved in lipid metabolism and transport. In conclusion, this study defines gene expression programs that build scutate scales and interscale epidermis of birds and reveals evolutionarily conserved keratinocyte differentiation genes.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Alibardi L. Adaptation to the land: The skin of reptiles in comparison to that of amphibians and endotherm amniotes. J. Exp. Zool. B Mol. Dev. Evol. 2003;298:12–41. - PubMed
-
- Sawyer, R.H., Knapp, L.W. & O’Guin, W.M. The skin of birds. Epidermis, dermis and appendages. In: Biology of the Integument (ed. Bereiter-Hahn, J., Matoltsy, A.G. & Richards, K.S.) 194–238 (Springer, 1986).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

