Clinical Outcomes of Patients with Combined Idiopathic Pulmonary Fibrosis and Emphysema in the IPF-PRO Registry
- PMID: 34997268
- PMCID: PMC8881259
- DOI: 10.1007/s00408-021-00506-x
Clinical Outcomes of Patients with Combined Idiopathic Pulmonary Fibrosis and Emphysema in the IPF-PRO Registry
Abstract
Purpose: To assess the impact of concomitant emphysema on outcomes in patients with idiopathic pulmonary fibrosis (IPF).
Methods: The IPF-PRO Registry is a US registry of patients with IPF. The presence of combined pulmonary fibrosis and emphysema (CPFE) at enrollment was determined by investigators' review of an HRCT scan. Associations between emphysema and clinical outcomes were analyzed using Cox proportional hazards models.
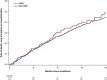
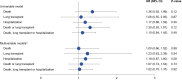
Results: Of 934 patients, 119 (12.7%) had CPFE. Compared with patients with IPF alone, patients with CPFE were older (median 72 vs 70 years); higher proportions were current/former smokers (88.2% vs 63.7%), used oxygen with activity (49.6% vs 31.9%) or at rest (30.8% vs 18.4%), had congestive heart failure (13.6% vs 4.8%) and had prior respiratory hospitalization (25.0% vs 16.7%); they had higher FVC (median 71.8 vs 69.4% predicted) and lower DLco (median 35.3 vs 43.6% predicted). In patients with CPFE and IPF alone, respectively, at 1 year, rates of death or lung transplant were 17.5% (95% CI: 11.7, 25.8) and 11.2% (9.2, 13.6) and rates of hospitalization were 21.6% (14.6, 29.6) and 20.6% (17.9, 23.5). There were no significant associations between emphysema and any outcome after adjustment for baseline variables. No baseline variable predicted outcomes better in IPF alone than in CPFE.
Conclusion: Approximately 13% of patients in the IPF-PRO Registry had CPFE. Physiologic characteristics and comorbidities of patients with CPFE differed from those of patients with IPF alone, but the presence of emphysema did not drive outcomes after adjustment for baseline covariates.
Trial registration: ClinicalTrials.gov, NCT01915511; registered August 5, 2013.
Keywords: Hospitalization; Interstitial lung disease; Mortality; Pulmonary fibrosis; Respiratory function tests.
© 2022. The Author(s).
Conflict of interest statement
HJK has no disclosures. LDS, MLN, ASH are employees of the Duke Clinical Research Institute (DCRI), which receives funding support from Boehringer Ingelheim Pharmaceuticals, Inc, to co-ordinate the IPF-PRO/ILD-PRO Registry. DLH reports research fees from Boehringer Ingelheim. LDM reports personal fees from Boehringer Ingelheim and Genentech. SB is an employee of Boehringer Ingelheim Pharmaceuticals, Inc. TBL was an employee of Boehringer Ingelheim Pharmaceuticals, Inc at the time this research was conducted. DAC reports personal fees from Boehringer Ingelheim and Genentech.
Figures
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

