B-cell intrinsic and extrinsic signals that regulate central tolerance of mouse and human B cells
- PMID: 34997597
- PMCID: PMC8986553
- DOI: 10.1111/imr.13062
B-cell intrinsic and extrinsic signals that regulate central tolerance of mouse and human B cells
Abstract
The random recombination of immunoglobulin V(D)J gene segments produces unique IgM antibodies that serve as the antigen receptor for each developing B cell. Hence, the newly formed B cell repertoire is comprised of a variety of specificities that display a range of reactivity with self-antigens. Newly generated IgM+ immature B cells that are non-autoreactive or that bind self-antigen with low avidity are licensed to leave the bone marrow with their intact antigen receptor and to travel via the blood to the peripheral lymphoid tissue for further selection and maturation. In contrast, clones with medium to high avidity for self-antigen remain within the marrow and undergo central tolerance, a process that revises their antigen receptor or eliminates the autoreactive B cell altogether. Thus, central B cell tolerance is critical for reducing the autoreactive capacity and avidity for self-antigen of our circulating B cell repertoire. Bone marrow cultures and mouse models have been instrumental for understanding the mechanisms that regulate the selection of bone marrow B cells. Here, we review recent studies that have shed new light on the contribution of the ERK, PI3K, and CXCR4 signaling pathways in the selection of mouse and human immature B cells that either bind or do not bind self-antigen.
Keywords: B cell tolerance; CXCR4; ERK; FOXO1; PI3K; autoimmunity; receptor editing.
© 2022 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
Conflicts of interest
The authors declare no conflicts of interest.
Figures


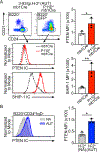
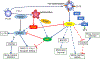
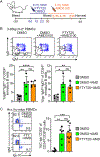

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

