Iron dysregulation in COVID-19 and reciprocal evolution of SARS-CoV-2: Natura nihil frustra facit
- PMID: 34997606
- PMCID: PMC9015563
- DOI: 10.1002/jcb.30207
Iron dysregulation in COVID-19 and reciprocal evolution of SARS-CoV-2: Natura nihil frustra facit
Abstract
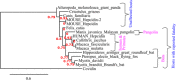
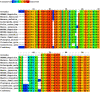
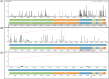
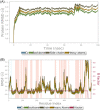
After more than a year of the COVID-19 pandemic, SARS-CoV-2 infection rates with newer variants continue to devastate much of the world. Global healthcare systems are overwhelmed with high positive patient numbers. Silent hypoxia accompanied by rapid deterioration and some cases with septic shock is responsible for COVID-19 mortality in many hospitalized patients. There is an urgent need to further understand the relationships and interplay with human host components during pathogenesis and immune evasion strategies. Currently, acquired immunity through vaccination or prior infection usually provides sufficient protection against the emerging variants of SARS-CoV-2 except Omicron variant requiring recent booster. New strains have shown higher viral loads and greater transmissibility with more severe disease presentations. Notably, COVID-19 has a peculiar prognosis in severe patients with iron dysregulation and hypoxia which is still poorly understood. Studies have shown abnormally low serum iron levels in severe infection but a high iron overload in lung fibrotic tissue. Data from our in-silico structural analysis of the spike protein sequence along with host proteolysis processing suggests that the viral spike protein fragment mimics Hepcidin and is resistant to the major human proteases. This functional spike-derived peptide dubbed "Covidin" thus may be intricately involved with host ferroportin binding and internalization leading to dysregulated host iron metabolism. Here, we propose the possible role of this potentially allogenic mimetic hormone corresponding to severe COVID-19 immunopathology and illustrate that this molecular mimicry is responsible for a major pathway associated with severe disease status. Furthermore, through 3D molecular modeling and docking followed by MD simulation validation, we have unraveled the likely role of Covidin in iron dysregulation in COVID-19 patients. Our meta-analysis suggests the Hepcidin mimetic mechanism is highly conserved among its host range as well as among all new variants to date including Omicron. Extensive analysis of current mutations revealed that new variants are becoming alarmingly more resistant to selective human proteases associated with host defense.
Keywords: MD simulations; evolved variants; ferritin-transferrin paradox; host proteases; hypoxia; iron homeostasis.
© 2022 Wiley Periodicals LLC.
Conflict of interest statement
The authors declare that there are no conflict of interests.
Figures






Comment in
-
Covidin, a possible new player between hepcidin and ferroportin in hypoxia and inflammation caused by COVID-19.J Cell Biochem. 2022 Nov;123(11):1701-1703. doi: 10.1002/jcb.30246. Epub 2022 Apr 5. J Cell Biochem. 2022. PMID: 35384033 No abstract available.
References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

