Cannabinol inhibits oxytosis/ferroptosis by directly targeting mitochondria independently of cannabinoid receptors
- PMID: 34999187
- PMCID: PMC8840979
- DOI: 10.1016/j.freeradbiomed.2022.01.001
Cannabinol inhibits oxytosis/ferroptosis by directly targeting mitochondria independently of cannabinoid receptors
Erratum in
-
Corrigendum to "Cannabinol inhibits oxytosis/ferroptosis by directly targeting mitochondria independently of cannabinoid receptors" [Free Radic. Biol. Med. 180 (2020 Feb 20) 33-51].Free Radic Biol Med. 2022 May 1;184:16. doi: 10.1016/j.freeradbiomed.2022.03.022. Epub 2022 Apr 2. Free Radic Biol Med. 2022. PMID: 35381547 Free PMC article. No abstract available.
Abstract
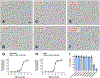
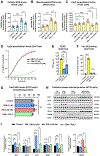
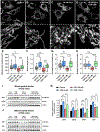
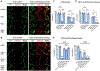
The oxytosis/ferroptosis regulated cell death pathway recapitulates many features of mitochondrial dysfunction associated with the aging brain and has emerged as a potential key mediator of neurodegeneration. It has thus been proposed that the oxytosis/ferroptosis pathway can be used to identify novel drug candidates for the treatment of age-associated neurodegenerative diseases that act by preserving mitochondrial function. Previously, we identified cannabinol (CBN) as a potent neuroprotector. Here, we demonstrate that not only does CBN protect nerve cells from oxytosis/ferroptosis in a manner that is dependent on mitochondria and it does so independently of cannabinoid receptors. Specifically, CBN directly targets mitochondria and preserves key mitochondrial functions including redox regulation, calcium uptake, membrane potential, bioenergetics, biogenesis, and modulation of fusion/fission dynamics that are disrupted following induction of oxytosis/ferroptosis. These protective effects of CBN are at least partly mediated by the promotion of endogenous antioxidant defenses and the activation of AMP-activated protein kinase (AMPK) signaling. Together, our data highlight the potential of mitochondrially-targeted compounds such as CBN as novel oxytotic/ferroptotic inhibitors to rescue mitochondrial dysfunction as well as opportunities for the discovery and development of future neurotherapeutics.
Keywords: AMPK; Aging; Antioxidant defense; Cannabinoid; Mitochondrial dysfunction; Neurodegenerative disease; Neurotherapeutics; Oxytosis/ferroptosis.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest
The authors declare no conflicts of interest.
Figures




References
-
- Hou Y, Dan X, Babbar M, Wei Y, Hasselbalch SG, Croteau DL, Bohr VA, Ageing as a risk factor for neurodegenerative disease, Nat. Rev. Neurol 15(10) (2019) 565–581. - PubMed
-
- Lin MT, Beal MF, Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases, Nature 443(7113) (2006) 787–795. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

