High-dose ivermectin for early treatment of COVID-19 (COVER study): a randomised, double-blind, multicentre, phase II, dose-finding, proof-of-concept clinical trial
- PMID: 34999239
- PMCID: PMC8734085
- DOI: 10.1016/j.ijantimicag.2021.106516
High-dose ivermectin for early treatment of COVID-19 (COVER study): a randomised, double-blind, multicentre, phase II, dose-finding, proof-of-concept clinical trial
Abstract
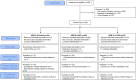
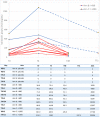
High concentrations of ivermectin demonstrated antiviral activity against SARS-CoV-2 in vitro. The aim of this study was to assess the safety and efficacy of high-dose ivermectin in reducing viral load in individuals with early SARS-CoV-2 infection. This was a randomised, double-blind, multicentre, phase II, dose-finding, proof-of-concept clinical trial. Participants were adults recently diagnosed with asymptomatic/oligosymptomatic SARS-CoV-2 infection. Exclusion criteria were: pregnant or lactating women; CNS disease; dialysis; severe medical condition with prognosis <6 months; warfarin treatment; and antiviral/chloroquine phosphate/hydroxychloroquine treatment. Participants were assigned (ratio 1:1:1) according to a randomised permuted block procedure to one of the following arms: placebo (arm A); single-dose ivermectin 600 μg/kg plus placebo for 5 days (arm B); and single-dose ivermectin 1200 μg/kg for 5 days (arm C). Primary outcomes were serious adverse drug reactions (SADRs) and change in viral load at Day 7. From 31 July 2020 to 26 May 2021, 32 participants were randomised to arm A, 29 to arm B and 32 to arm C. Recruitment was stopped on 10 June because of a dramatic drop in cases. The safety analysis included 89 participants and the change in viral load was calculated in 87 participants. No SADRs were registered. Mean (S.D.) log10 viral load reduction was 2.9 (1.6) in arm C, 2.5 (2.2) in arm B and 2.0 (2.1) in arm A, with no significant differences (P = 0.099 and 0.122 for C vs. A and B vs. A, respectively). High-dose ivermectin was safe but did not show efficacy to reduce viral load.
Keywords: COVID-19; Ivermectin; Randomised controlled trial; SARS-CoV-2.
Copyright © 2022 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures
Comment in
-
Viral Load Reduction and High-Dose Ivermectin in Early Treatment: A Reappraisal.Int J Antimicrob Agents. 2022 May;59(5):106575. doi: 10.1016/j.ijantimicag.2022.106575. Epub 2022 Mar 20. Int J Antimicrob Agents. 2022. PMID: 35322790 No abstract available.
References
-
- Mallapaty S. COVID vaccines slash viral spread—but Delta is an unknown. Nature. 2021;596:17–18. - PubMed

