Clinically Meaningful Benefit in Women with Hypoactive Sexual Desire Disorder Treated with Flibanserin
- PMID: 34999484
- PMCID: PMC8847820
- DOI: 10.1016/j.esxm.2021.100476
Clinically Meaningful Benefit in Women with Hypoactive Sexual Desire Disorder Treated with Flibanserin
Abstract
Background: The efficacy of flibanserin in treating hypoactive sexual desire disorder (HSDD) is based upon statistically significant improvements in sexual desire, satisfying sexual events, and distress. However, clinically meaningful benefit has not been well characterized.
Aim: Evaluate clinically meaningful benefit of flibanserin.
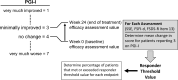
Methods: Data were pooled from 3 pivotal trials evaluating flibanserin 100 mg qhs in premenopausal women (flibanserin, n = 1192; placebo, n = 1215). Flibanserin trial data in postmenopausal women (flibanserin, n = 450; placebo, n = 476) were analyzed separately. Clinically meaningful benefit was evaluated by the Patient Global Impression of Improvement (PGI-I). Responders were determined through anchor-based analyses that used the PGI-I for key efficacy endpoints: satisfying sexual events (SSE), desire domain of the Female Sexual Function Index (FSFI-d), and distress associated with decreased sexual desire (FSDS-R13). Odds ratios were calculated to assess effect size and Kaplan-Meier analyses were performed to estimate onset time for treatment benefit.
Outcomes: PGI-I, anchor-based analyses for key efficacy endpoints (SSE, FSFI-d, FSDS-R13), odds ratios, onset time for treatment benefit.
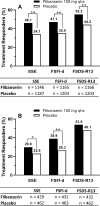
Results: Based on the PGI-I, more patients reported clinically meaningful benefit with flibanserin treatment versus placebo (49.8% vs 33.6%, premenopausal cohort; 40.5% vs 28.7%, postmenopausal cohort). In anchor-based analyses, responder rates were significantly higher for premenopausal women on flibanserin (46.1%-55.2%) than placebo (34.1%-44.2%) for all 3 key efficacy endpoints (P < .0001). Responder rates for postmenopausal women on flibanserin were higher compared to placebo for SSE (29.8% vs 22.9%; P = .015) and FSFI-d (38.9% vs 26.3%; P = .0001). Odds ratios for key endpoints indicated that premenopausal women were 2.0-2.4 times as likely to be responders with flibanserin treatment compared to placebo. Postmenopausal women were 1.6 times as likely to be responders with flibanserin for FSFI-d. Kaplan-Meier analyses indicated significant separation between flibanserin and placebo for the key endpoints in both premenopausal and postmenopausal cohorts (log-rank tests P < .01) with earlier median response times among patients receiving flibanserin.
Clinical implications: Patient-reported benefit assessments such as the PGI-I capture the patient's perspective and may be a useful approach in assessing overall clinical meaningfulness for sexual dysfunction therapies.
Strengths and limitations: Strengths include a well-powered study with large enrollment, use of validated instruments, and self-assessment of treatment benefit. Limitations include pooling of trial data in premenopausal women with slightly different study designs and use of an endpoint (SSE) indirectly related to HSDD.
Conclusion: Assessment of clinically meaningful benefit and additional responder analyses provide further support for flibanserin's efficacy beyond numerical improvements in endpoint measures. Simon JA, Clayton AH, Kim NN, et al. Clinically Meaningful Benefit in Women with Hypoactive Sexual Desire Disorder Treated with Flibanserin. Sex Med 2022;10:100476.
Keywords: anchor-based analysis; clinically meaningful benefit; flibanserin; hypoactive sexual desire disorder.
Copyright © 2021 International Society for Sexual Medicine. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Parish SJ, Goldstein AT, Goldstein SW, et al. Toward a more evidence-based nosology and nomenclature for female sexual dysfunctions—part II. J Sex Med. 2016;13:1888–1906. - PubMed
-
- Goldstein I, Kim NN, Clayton AH, et al. Hypoactive sexual desire disorder: International Society for the Study of Women's Sexual Health (ISSWSH) expert consensus panel review. Mayo Clin Proc. 2017;92:114–128. - PubMed
-
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems (ICD), 11th Revision. Available at: https://icd.who.int/browse11/l-m/en. Accessed June 1, 2021
-
- Addyi® (flibanserin) [package insert]. Raleigh, NC: Sprout Pharmaceuticals, Inc.; October 2019.
-
- Addyi® (flibanserin) [product monograph]. Montreal, QC, CANADA: Searchlight Pharma, Inc.; January 2021.

