Prospective evaluation of a breast-cancer risk model integrating classical risk factors and polygenic risk in 15 cohorts from six countries
- PMID: 34999890
- PMCID: PMC8743128
- DOI: 10.1093/ije/dyab036
Prospective evaluation of a breast-cancer risk model integrating classical risk factors and polygenic risk in 15 cohorts from six countries
Abstract
Background: Rigorous evaluation of the calibration and discrimination of breast-cancer risk-prediction models in prospective cohorts is critical for applications under clinical guidelines. We comprehensively evaluated an integrated model incorporating classical risk factors and a 313-variant polygenic risk score (PRS) to predict breast-cancer risk.
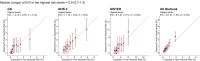
Methods: Fifteen prospective cohorts from six countries with 239 340 women (7646 incident breast-cancer cases) of European ancestry aged 19-75 years were included. Calibration of 5-year risk was assessed by comparing expected and observed proportions of cases overall and within risk categories. Risk stratification for women of European ancestry aged 50-70 years in those countries was evaluated by the proportion of women and future cases crossing clinically relevant risk thresholds.
Results: Among women <50 years old, the median (range) expected-to-observed ratio for the integrated model across 15 cohorts was 0.9 (0.7-1.0) overall and 0.9 (0.7-1.4) at the highest-risk decile; among women ≥50 years old, these were 1.0 (0.7-1.3) and 1.2 (0.7-1.6), respectively. The proportion of women identified above a 3% 5-year risk threshold (used for recommending risk-reducing medications in the USA) ranged from 7.0% in Germany (∼841 000 of 12 million) to 17.7% in the USA (∼5.3 of 30 million). At this threshold, 14.7% of US women were reclassified by adding the PRS to classical risk factors, with identification of 12.2% of additional future cases.
Conclusion: Integrating a 313-variant PRS with classical risk factors can improve the identification of European-ancestry women at elevated risk who could benefit from targeted risk-reducing strategies under current clinical guidelines.
Keywords: Breast cancer; iCARE; model validation; polygenic risk score; risk prediction; risk stratification.
Published by Oxford University Press on behalf of the International Epidemiological Association 2021. This work is written by US Government employees and is in the public domain in the US.
Figures





Comment in
-
Commentary: Polygenic risk for breast cancer: in search for potential clinical utility.Int J Epidemiol. 2022 Jan 6;50(6):1911-1913. doi: 10.1093/ije/dyab230. Epub 2021 Oct 31. Int J Epidemiol. 2022. PMID: 34999886 Free PMC article. No abstract available.
References
-
- Visvanathan K, Fabian CJ, Bantug E. et al. Use of endocrine therapy for breast cancer risk reduction: ASCO clinical practice guideline update. J Clin Oncol 37:JCO.19.01472. - PubMed
-
- Owens DK, Davidson KW, Krist AH, et al.; US Preventive Services Task Force. Medication use to reduce risk of breast cancer: US preventive services task force recommendation statement. JAMA 2019;322:857–67. - PubMed
-
- Familial breast cancer: classification, care and managing breast cancer and related risks in people with a family history of breast cancer. The National Institute for Health and Care Excellence (NICE), 2013. http://nice.org.uk/guidance/cg164 (8 March 2021, date last accessed). - PubMed
-
- Cintolo-Gonzalez JA, Braun D, Blackford AL. et al. Breast cancer risk models: a comprehensive overview of existing models, validation, and clinical applications. Breast Cancer Res Treat 2017;164:263–84. - PubMed
Publication types
MeSH terms
Grants and funding
- MR/M012190/1/MRC_/Medical Research Council/United Kingdom
- R01 CA047988 /CA/NCI NIH HHS/United States
- UM1 CA182913 /CA/NCI NIH HHS/United States
- MR/N003284/1/MRC_/Medical Research Council/United Kingdom
- 20861/CRUK_/Cancer Research UK/United Kingdom
- G1000143/MRC_/Medical Research Council/United Kingdom
- 14136/CRUK_/Cancer Research UK/United Kingdom
- G0401527/MRC_/Medical Research Council/United Kingdom
- U19 CA148112/CA/NCI NIH HHS/United States
- U01 CA182913/CA/NCI NIH HHS/United States
- C1287/A16563/CRUK_/Cancer Research UK/United Kingdom
- R01 CA128978/CA/NCI NIH HHS/United States
- C1287/A10118/CRUK_/Cancer Research UK/United Kingdom

