Combination immunotherapy including OncoVEXmGMCSF creates a favorable tumor immune micro-environment in transgenic BRAF murine melanoma
- PMID: 34999916
- PMCID: PMC10991384
- DOI: 10.1007/s00262-021-03088-y
Combination immunotherapy including OncoVEXmGMCSF creates a favorable tumor immune micro-environment in transgenic BRAF murine melanoma
Abstract
Talimogene Laherparepvec (OncoVEXmGMCSF), an oncolytic virus, immune checkpoint inhibitor anti-programmed cell death protein 1 (anti-PD1), and BRAF inhibition (BRAFi), are all clinically approved for treatment of melanoma patients and are effective through diverse mechanisms of action. Individually, these therapies also have an effect on the tumor immune microenvironment (TIME). Evaluating the combination effect of these three therapies on the TIME can help determine when combination therapy is most appropriate for further study. In this study, we use a transgenic murine melanoma model (Tyr::CreER; BRAFCA/+; PTENflox/flox), to evaluate the TIME in response to combinations of BRAFi, anti-PD1, and OncoVEXmGMCSF. We find that mice treated with the triple combination BRAFi + anti-PD1 + OncoVEXmGMCSF have decreased tumor growth compared to BRAFi alone and prolonged survival compared to control. Flow cytometry shows an increase in percent CD8 + /CD3 + cytotoxic T Lymphocytes (CTLs) and a decrease in percent FOXP3 + /CD4 + T regulatory cells (Tregs) in tumors treated with OncoVEXmGMCSF compared to mice not treated with OncoVEXmGMCSF. Immunogenomic analysis at 30d post-treatment shows an increase in Th1 and interferon-related genes in mice receiving OncoVEXmGMCSF + BRAFi. In summary, treatment with combination BRAFi + anti-PD1 + OncoVEXmGMCSF is more effective than any single treatment in controlling tumor growth, and groups receiving OncoVEXmGMCSF had more tumoral infiltration of CTLs and less intratumoral Tregs in the TIME. This study provides rational basis to combine targeted agents, oncolytic viral therapy, and checkpoint inhibitors in the treatment of melanoma.
Keywords: Immunotherapy; Melanoma; Microenvironment; Oncolytic virus; Tumor-infiltrating lymphocyte.
© 2021. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
Authors have no disclosures.
Figures

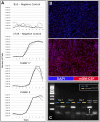


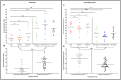

References
-
- Dummer R, Ascierto PA, Gogas HJ, et al. Overall survival in patients with BRAF-mutant melanoma receiving encorafenib plus binimetinib versus vemurafenib or encorafenib (COLUMBUS): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2018;19:1315–1327. doi: 10.1016/S1470-2045(18)30497-2. - DOI - PubMed
-
- Sun L, Funchain P, Song JM, Rayman P, Tannenbaum C, Ko J, McNamara M, Marcela Diaz-Montero C, Gastman B. Talimogene Laherparepvec combined with anti-PD-1 based immunotherapy for unresectable stage III-IV melanoma: a case series. J Immunother Cancer. 2018;6:36. doi: 10.1186/s40425-018-0337-7. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

