Hypoxia-inducible factor-1 alpha expression is induced by IL-2 via the PI3K/mTOR pathway in hypoxic NK cells and supports effector functions in NKL cells and ex vivo expanded NK cells
- PMID: 34999917
- PMCID: PMC9294031
- DOI: 10.1007/s00262-021-03126-9
Hypoxia-inducible factor-1 alpha expression is induced by IL-2 via the PI3K/mTOR pathway in hypoxic NK cells and supports effector functions in NKL cells and ex vivo expanded NK cells
Abstract
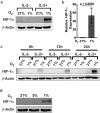
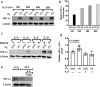
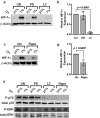
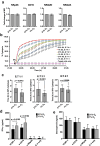
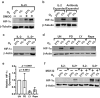
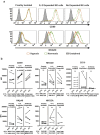
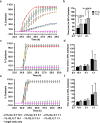
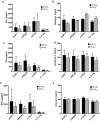
Natural killer (NK) cells are cytotoxic innate lymphocytes that are specialized to kill tumor cells. NK cells are responsive to the primary cytokine IL-2 in the tumor microenvironment (TME), to activate its effector functions against tumors. Despite their inherent ability to kill tumor cells, dysfunctional NK cells observed within advanced solid tumors are associated with poor patient survival. Hypoxia in the TME is a major contributor to immune evasion in solid tumors that could contribute to impaired NK cell function. HIF-1α is a nodal regulator of hypoxia in driving the adaptive cellular responses to changes in oxygen concentrations. Whether HIF-1α is expressed in hypoxic NK cells in the context of IL-2 and whether its expression regulates NK cell effector function are unclear. Here, we report that freshly isolated NK cells from human peripheral blood in hypoxia could not stabilize HIF-1α protein coincident with impaired anti-tumor cytotoxicity. However, ex vivo expansion of these cells restored HIF-1α levels in hypoxia to promote antitumor cytotoxic functions. Similarly, the human NK cell line NKL expressed HIF-1α upon IL-2 stimulation in hypoxia and exhibited improved anti-tumor cytotoxicity and IFN-γ secretion. We found that ex vivo expanded human NK cells and NKL cells required the concerted activation of PI3K/mTOR pathway initiated by IL-2 signaling in combination with hypoxia for HIF-1α stabilization. These findings highlight that HIF-1α stabilization in hypoxia maximizes NK cell effector function and raises the prospect of NK cells as ideal therapeutic candidates for solid tumors.
Keywords: HIF-1α; Hypoxia; IL-2; Natural killer cells.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Similar articles
-
Inflammatory interferon activates HIF-1α-mediated epithelial-to-mesenchymal transition via PI3K/AKT/mTOR pathway.J Exp Clin Cancer Res. 2018 Mar 27;37(1):70. doi: 10.1186/s13046-018-0730-6. J Exp Clin Cancer Res. 2018. PMID: 29587825 Free PMC article.
-
Inflammatory stimulation and hypoxia cooperatively activate HIF-1{alpha} in bronchial epithelial cells: involvement of PI3K and NF-{kappa}B.Am J Physiol Lung Cell Mol Physiol. 2010 May;298(5):L660-9. doi: 10.1152/ajplung.00394.2009. Epub 2010 Feb 5. Am J Physiol Lung Cell Mol Physiol. 2010. PMID: 20139176
-
Characterization of HIF-1α Knockout Primary Human Natural Killer Cells Including Populations in Allogeneic Glioblastoma.Int J Mol Sci. 2024 May 28;25(11):5896. doi: 10.3390/ijms25115896. Int J Mol Sci. 2024. PMID: 38892084 Free PMC article.
-
[Hypoxia-inducible factor-1alpha: a promising target for tumor therapy].Ai Zheng. 2009 Jul;28(7):775-82. doi: 10.5732/cjc.008.10770. Ai Zheng. 2009. PMID: 19624909 Review. Chinese.
-
Role of HIF-1α in the Responses of Tumors to Radiotherapy and Chemotherapy.Cancer Res Treat. 2025 Jan;57(1):1-10. doi: 10.4143/crt.2024.255. Epub 2024 Jun 5. Cancer Res Treat. 2025. PMID: 38853541 Free PMC article. Review.
Cited by
-
The role of hypoxic microenvironment in autoimmune diseases.Front Immunol. 2024 Nov 7;15:1435306. doi: 10.3389/fimmu.2024.1435306. eCollection 2024. Front Immunol. 2024. PMID: 39575238 Free PMC article. Review.
-
Emerging role of hypoxia-inducible factor-1α in inflammatory autoimmune diseases: A comprehensive review.Front Immunol. 2023 Jan 25;13:1073971. doi: 10.3389/fimmu.2022.1073971. eCollection 2022. Front Immunol. 2023. PMID: 36761171 Free PMC article. Review.
-
Advances in metabolic reprogramming of NK cells in the tumor microenvironment on the impact of NK therapy.J Transl Med. 2024 Mar 3;22(1):229. doi: 10.1186/s12967-024-05033-w. J Transl Med. 2024. PMID: 38433193 Free PMC article. Review.
-
Unraveling the role of HIF-1α in sepsis: from pathophysiology to potential therapeutics-a narrative review.Crit Care. 2024 Mar 27;28(1):100. doi: 10.1186/s13054-024-04885-4. Crit Care. 2024. PMID: 38539163 Free PMC article. Review.
-
STAT5 Is Necessary for the Metabolic Switch Induced by IL-2 in Cervical Cancer Cell Line SiHa.Int J Mol Sci. 2024 Jun 21;25(13):6835. doi: 10.3390/ijms25136835. Int J Mol Sci. 2024. PMID: 38999946 Free PMC article.
References
-
- Nakamura H, Makino Y, Okamoto K, Poellinger L, Ohnuma K, Morimoto C, Tanaka H. TCR engagement increases hypoxia-inducible factor-1 alpha protein synthesis via rapamycin-sensitive pathway under hypoxic conditions in human peripheral T cells. J Immunol. 2005;174:7592–7599. doi: 10.4049/jimmunol.174.12.7592. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

