Bioactive fish collagen peptides weaken intestinal inflammation by orienting colonic macrophages phenotype through mannose receptor activation
- PMID: 34999930
- PMCID: PMC9106617
- DOI: 10.1007/s00394-021-02787-7
Bioactive fish collagen peptides weaken intestinal inflammation by orienting colonic macrophages phenotype through mannose receptor activation
Abstract
Purpose: Particular interest is now given to the potential of dietary supplements as alternative non-pharmacological approaches in intestinal inflammation handling. In this aim, this study evaluates the efficiency of fish collagen peptides, Naticol®Gut, on colonic inflammation.
Methods: Wild type and Mannose receptor-deficient in the myeloid lineage C57BL/6 mice were administered with Dextran Sodium Sulfate (DSS), Naticol®Gut, DSS, and Naticol®Gut or only water for 4 or 8 days. Inflammatory status was evaluated by establishing macroscopic and microscopic scores, by measuring cytokine and calprotectin production by ELISA and the myeloperoxidase activity by chemiluminescence. Colonic macrophages were phenotyped by measuring mRNA levels of specific markers of inflammation and oxidative status. Colonic immune populations and T-cell activation profiles were determined by flow cytometry. Mucosa-associated gut microbiota assessment was undertaken by qPCR. The phenotype of human blood monocytes from inflammatory bowel disease (IBD) subjects was characterized by RT-qPCR and flow cytometry and their oxidative activity by chemiluminescence.
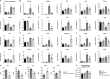
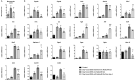
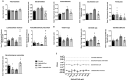
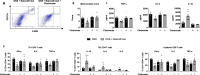
Results: Naticol®Gut-treated DSS mice showed attenuated colonic inflammation compared to mice that were only exposed to DSS. Naticol®Gut activity was displayed through its ability to orient the polarization of colonic macrophage towards an anti-inflammatory and anti-oxidant phenotype after its recognition by the mannose receptor. Subsequently, Naticol®Gut delivery modulated CD4 T cells in favor of a Th2 response and dampened CD8 T-cell activation. This immunomodulation resulted in an intestinal eubiosis. In human monocytes from IBD subjects, the treatment with Naticol®Gut also restored an anti-inflammatory and anti-oxidant phenotype.
Conclusion: Naticol®Gut acts as a protective agent against colitis appearing as a new functional food and an innovative and complementary approach in gut health.
Keywords: Collagen; Gut inflammation; Innate and adaptive immunity; Mannose receptor; Microbiota.
© 2022. The Author(s).
Conflict of interest statement
This work was supported by a grant from CIFRE-Association Nationale de la Recherche et de la Technologie (ANRT) awarded to Ms. Rahabi (2016/1443) and from the Weishardt Group.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

