Impaired neutralisation of SARS-CoV-2 delta variant in vaccinated patients with B cell chronic lymphocytic leukaemia
- PMID: 35000597
- PMCID: PMC8743056
- DOI: 10.1186/s13045-021-01219-7
Impaired neutralisation of SARS-CoV-2 delta variant in vaccinated patients with B cell chronic lymphocytic leukaemia
Abstract
Background: Immune suppression is a clinical feature of chronic lymphocytic leukaemia (CLL), and patients show increased vulnerability to SARS-CoV-2 infection and suboptimal antibody responses.
Method: We studied antibody responses in 500 patients following dual COVID-19 vaccination to assess the magnitude, correlates of response, stability and functional activity of the spike-specific antibody response with two different vaccine platforms.
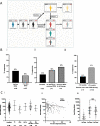
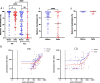
Results: Spike-specific seroconversion post-vaccine was seen in 67% of patients compared to 100% of age-matched controls. Amongst responders, titres were 3.7 times lower than the control group. Antibody responses showed a 33% fall over the next 4 months. The use of an mRNA (n = 204) or adenovirus-based (n = 296) vaccine platform did not impact on antibody response. Male gender, BTKi therapy, prophylactic antibiotics use and low serum IgA/IgM were predictive of failure to respond. Antibody responses after CD20-targeted immunotherapy recovered 12 months post treatment. Post-vaccine sera from CLL patients with Spike-specific antibody response showed markedly reduced neutralisation of the SARS-CoV-2 delta variant compared to healthy controls. Patients with previous natural SARS-CoV-2 infection showed equivalent antibody levels and function as healthy donors after vaccination.
Conclusions: These findings demonstrate impaired antibody responses following dual COVID-19 vaccination in patients with CLL and further define patient risk groups. Furthermore, humoural protection against the globally dominant delta variant is markedly impaired in CLL patients and indicates the need for further optimisation of immune protection in this patient cohort.
Keywords: Antibody; CLL; COVID; Leukaemia; SARS-CoV-2; Vaccination.
© 2021. The Author(s).
Conflict of interest statement
There are no competing interests to declare.
Figures

References
-
- Scarfò L, Chatzikonstantinou T, Rigolin GM, Quaresmini G, Motta M, Vitale C, et al. COVID-19 severity and mortality in patients with chronic lymphocytic leukemia: a joint study by ERIC, the European Research Initiative on CLL, and CLL Campus. Leukemia. 2020;34(9):2354–2363. doi: 10.1038/s41375-020-0959-x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

