Sleep and circadian rhythms in Parkinson's disease and preclinical models
- PMID: 35000606
- PMCID: PMC8744293
- DOI: 10.1186/s13024-021-00504-w
Sleep and circadian rhythms in Parkinson's disease and preclinical models
Abstract
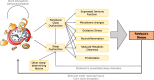
The use of animals as models of human physiology is, and has been for many years, an indispensable tool for understanding the mechanisms of human disease. In Parkinson's disease, various mouse models form the cornerstone of these investigations. Early models were developed to reflect the traditional histological features and motor symptoms of Parkinson's disease. However, it is important that models accurately encompass important facets of the disease to allow for comprehensive mechanistic understanding and translational significance. Circadian rhythm and sleep issues are tightly correlated to Parkinson's disease, and often arise prior to the presentation of typical motor deficits. It is essential that models used to understand Parkinson's disease reflect these dysfunctions in circadian rhythms and sleep, both to facilitate investigations into mechanistic interplay between sleep and disease, and to assist in the development of circadian rhythm-facing therapeutic treatments. This review describes the extent to which various genetically- and neurotoxically-induced murine models of Parkinson's reflect the sleep and circadian abnormalities of Parkinson's disease observed in the clinic.
Keywords: Non-motor symptoms; Parkinson’s disease; RBD; circadian; insomnia; rapid eye movement; research models; sleep; sleep behavior disorder.
© 2021. The Author(s).
Conflict of interest statement
No competing interests
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

