Herd Immunity Effects in Cost-Effectiveness Analyses among Low- and Middle-Income Countries
- PMID: 35001292
- PMCID: PMC8743090
- DOI: 10.1007/s40258-021-00711-y
Herd Immunity Effects in Cost-Effectiveness Analyses among Low- and Middle-Income Countries
Abstract
Background: Herd immunity (HI) is a key benefit of vaccination programs, but the effects are not routinely included in cost-effectiveness analyses (CEAs).
Objective: This study investigated how the inclusion of HI in CEAs may influence the reported value of immunizations in low- and middle-income countries (LMICs) and illustrated the implications for COVID-19 immunization.
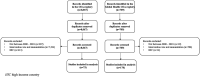
Methods: We reviewed immunization CEAs published from 2000 to 2018 focusing on LMICs using data from the Tufts Medical Center CEA Registries. We investigated the proportion of studies that included HI, the methods used, and the incremental cost-effectiveness ratios (ICERs) reported. When possible, we evaluated how ICERs would change with and without HI.
Results: Among the 243 immunization CEAs meeting inclusion criteria, 44 studies (18%) included HI. Of those studies, 11 (25%) used dynamic transmission models, whereas the remainder used static models. Sixteen studies allowed for ICER calculations with and without HI (n = 48 ratios). The inclusion of HI always resulted in more favorable ratios. In 20 cases (42%), adding HI decreased the ICERs enough to cross at least one or more common cost-effectiveness benchmarks for LMICs. Among pneumococcal vaccination studies, including HI in the analyses decreased seven of 24 ICERs enough to cross at least one cost-effectiveness benchmark.
Conclusion: The full value of immunization may be underestimated without considering a scenario in which HI is achieved. Given the evidence in pneumococcal CEAs, COVID-19 vaccine value assessments should aim to show ICERs with and without HI to inform decision-making in LMICs.
© 2021. The Author(s).
Conflict of interest statement
The authors report grants from the Gates Foundation during the conduct of this study. The authors are affiliated with the CEA Registry, which receives unrestricted sponsorships from a number of government, private foundation, and pharmaceutical industry sponsors (list is available at
Figures
Similar articles
-
Impact of vaccine herd-protection effects in cost-effectiveness analyses of childhood vaccinations. A quantitative comparative analysis.PLoS One. 2017 Mar 1;12(3):e0172414. doi: 10.1371/journal.pone.0172414. eCollection 2017. PLoS One. 2017. PMID: 28249046 Free PMC article.
-
Cost-effectiveness of rotavirus vaccination in children under five years of age in 195 countries: A meta-regression analysis.Vaccine. 2022 Jun 21;40(28):3903-3917. doi: 10.1016/j.vaccine.2022.05.042. Epub 2022 May 25. Vaccine. 2022. PMID: 35643565 Free PMC article.
-
Industry sponsorship bias in cost effectiveness analysis: registry based analysis.BMJ. 2022 Jun 22;377:e069573. doi: 10.1136/bmj-2021-069573. BMJ. 2022. PMID: 35732297 Free PMC article.
-
Adverse Event Costs and Cost-Effectiveness Analyses of Anticancer Drugs: A Systematic Review.JAMA Netw Open. 2025 May 1;8(5):e2512455. doi: 10.1001/jamanetworkopen.2025.12455. JAMA Netw Open. 2025. PMID: 40423968 Free PMC article.
-
The impact of indirect (herd) protection on the cost-effectiveness of pneumococcal conjugate vaccine.Clin Ther. 2008 Feb;30(2):341-57. doi: 10.1016/j.clinthera.2008.02.003. Clin Ther. 2008. PMID: 18343273 Review.
Cited by
-
Effectiveness, cost-effectiveness, and positive externalities of integrated chronic care for adults with major depressive disorder in Malawi (IC3D): a stepped-wedge, cluster-randomised, controlled trial.Lancet. 2024 Nov 9;404(10465):1823-1834. doi: 10.1016/S0140-6736(24)01809-9. Epub 2024 Oct 30. Lancet. 2024. PMID: 39488229 Clinical Trial.
-
Data-Related Challenges in Cost-Effectiveness Analyses of Vaccines.Appl Health Econ Health Policy. 2022 Jul;20(4):457-465. doi: 10.1007/s40258-022-00718-z. Epub 2022 Feb 9. Appl Health Econ Health Policy. 2022. PMID: 35138601 Free PMC article.
-
Development and validation of a rapid five-minute nucleic acid extraction method for respiratory viruses.Virol J. 2024 Aug 18;21(1):189. doi: 10.1186/s12985-024-02381-3. Virol J. 2024. PMID: 39155366 Free PMC article.
-
Economic evaluations of vaccines against respiratory infections in adults in Southeast Asia: A systematic review.Hum Vaccin Immunother. 2025 Dec;21(1):2528409. doi: 10.1080/21645515.2025.2528409. Epub 2025 Jul 15. Hum Vaccin Immunother. 2025. PMID: 40665593 Free PMC article.
-
Public Health and Economic Impact of Periodic COVID-19 Vaccination with BNT162b2 for Old Adults and High-Risk Patients in an Illustrative Prefecture of Japan: A Budget Impact Analysis.Infect Dis Ther. 2024 Oct;13(10):2155-2177. doi: 10.1007/s40121-024-01032-y. Epub 2024 Sep 10. Infect Dis Ther. 2024. PMID: 39254889 Free PMC article.
References
-
- Gargano LM, Hajjeh R, Cookson ST. Pneumonia prevention during a humanitarian emergency: cost-effectiveness of Haemophilus influenzae type b conjugate vaccine and pneumococcal conjugate vaccine in Somalia. Prehosp Disaster Med. 2015;30(4):402–411. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical