Estimation of the capillary level input function for dynamic contrast-enhanced MRI of the breast using a deep learning approach
- PMID: 35001423
- PMCID: PMC8852816
- DOI: 10.1002/mrm.29148
Estimation of the capillary level input function for dynamic contrast-enhanced MRI of the breast using a deep learning approach
Abstract
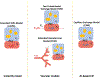
Purpose: To develop a deep learning approach to estimate the local capillary-level input function (CIF) for pharmacokinetic model analysis of DCE-MRI.
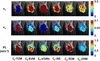
Methods: A deep convolutional network was trained with numerically simulated data to estimate the CIF. The trained network was tested using simulated lesion data and used to estimate voxel-wise CIF for pharmacokinetic model analysis of breast DCE-MRI data using an abbreviated protocol from women with malignant (n = 25) and benign (n = 28) lesions. The estimated parameters were used to build a logistic regression model to detect the malignancy.
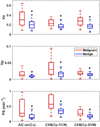
Result: The pharmacokinetic parameters estimated using the network-predicted CIF from our breast DCE data showed significant differences between the malignant and benign groups for all parameters. Testing the diagnostic performance with the estimated parameters, the conventional approach with arterial input function (AIF) showed an area under the curve (AUC) between 0.76 and 0.87, and the proposed approach with CIF demonstrated similar performance with an AUC between 0.79 and 0.81.
Conclusion: This study shows the feasibility of estimating voxel-wise CIF using a deep neural network. The proposed approach could eliminate the need to measure AIF manually without compromising the diagnostic performance to detect the malignancy in the clinical setting.
Keywords: arterial input function; breast cancer; capillary input function; deep learning; dynamic contrast enhanced MRI.
© 2022 International Society for Magnetic Resonance in Medicine.
Figures




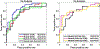
References
-
- Abdullah SS, Pialat JB, Wiart M, Duboeuf F, Mabrut JY, Bancel B, et al. Characterization of hepatocellular carcinoma and colorectal liver metastasis by means of perfusion MRI. Journal of Magnetic Resonance Imaging: An Official Journal of the International Society for Magnetic Resonance in Medicine. 2008;28(2):390–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

