Variable histopathology features of neuronal dyslamination in the cerebral neocortex adjacent to epilepsy-associated vascular malformations suggest complex pathogenesis of focal cortical dysplasia ILAE type IIIc
- PMID: 35001442
- PMCID: PMC9425012
- DOI: 10.1111/bpa.13052
Variable histopathology features of neuronal dyslamination in the cerebral neocortex adjacent to epilepsy-associated vascular malformations suggest complex pathogenesis of focal cortical dysplasia ILAE type IIIc
Abstract
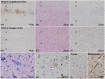
Focal cortical dysplasia type IIIc (FCD-IIIc) is histopathologically defined by the International League Against Epilepsy's classification scheme as abnormal cortical organization adjacent to epilepsy-associated vascular malformations (VM). However, the incidence of FCD-IIIc, its pathogenesis, or association with the epileptogenic condition remains to be clarified. We reviewed a retrospective series of surgical brain specimens from 14 epilepsy patients with leptomeningeal angiomatosis of Sturge-Weber syndrome (LMA-SWS; n = 6), cerebral cavernous malformations (CCM; n = 7), and an arteriovenous malformation (AVM; n = 1) to assess the histopathological spectrum of FCD-IIIc patterns in VM. FCD-IIIc was observed in all cases of LMA-SWS and was designated as cortical pseudolaminar sclerosis (CPLS). CPLS showed a common pattern of horizontally organized layer abnormalities, including neuronal cell loss and astrogliosis, either manifesting predominantly in cortical layer (L) 3 extending variably to deeper areas with or without further extension to L2 and/or L4. Another pattern was more localized, targeting mainly L4 with extension to L3 and/or L5. Abnormal cortical layering characterized by a fusion of L2 and L3 or L4-L6 was also noted in two LMA-SWS cases and the AVM case. No horizontal or vertical lamination abnormalities were observed in the specimens adjacent to the CCM, despite the presence of vascular congestion and dilated parenchymal veins in all VM. These findings suggest that FCD-IIIc depends on the type of the VM and developmental timing. We further conclude that FCD-IIIc represents a secondary lesion acquired during pre- and/or perinatal development rather than following a pathomechanism independent of LMA-SWS. Further studies will be necessary to address the selective vulnerability of the developing cerebral neocortex in LMA-SWS, including genetic, encephaloclastic, hemodynamic, or metabolic events.
Keywords: cerebral neocortex; epilepsy surgery; focal cortical dysplasia; neuron; pathogenesis; vascular malformation.
© 2022 The Authors. Brain Pathology published by John Wiley & Sons Ltd on behalf of International Society of Neuropathology.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures





Similar articles
-
Focal cortical dysplasia type IIIc associates with multiple cerebral cavernomas.Epilepsy Res. 2013 Nov;107(1-2):190-4. doi: 10.1016/j.eplepsyres.2013.07.006. Epub 2013 Aug 5. Epilepsy Res. 2013. PMID: 23968818
-
Sturge-Weber Syndrome Is Associated with Cortical Dysplasia ILAE Type IIIc and Excessive Hypertrophic Pyramidal Neurons in Brain Resections for Intractable Epilepsy.Brain Pathol. 2015 May;25(3):248-55. doi: 10.1111/bpa.12172. Epub 2014 Oct 30. Brain Pathol. 2015. PMID: 25040707 Free PMC article.
-
Neuropathological work-up of focal cortical dysplasias using the new ILAE consensus classification system - practical guideline article invited by the Euro-CNS Research Committee.Clin Neuropathol. 2011 Jul-Aug;30(4):164-77. doi: 10.5414/np300398. Clin Neuropathol. 2011. PMID: 21726501
-
Focal cortical dysplasia type 1.Brain Pathol. 2021 Jul;31(4):e12964. doi: 10.1111/bpa.12964. Brain Pathol. 2021. PMID: 34196986 Free PMC article. Review.
-
Surgical pathology of epilepsy-associated non-neoplastic cerebral lesions: a brief introduction with special reference to hippocampal sclerosis and focal cortical dysplasia.Neuropathology. 2013 Aug;33(4):442-58. doi: 10.1111/neup.12028. Epub 2013 Mar 27. Neuropathology. 2013. PMID: 23530853 Free PMC article. Review.
Cited by
-
The ILAE consensus classification of focal cortical dysplasia: An update proposed by an ad hoc task force of the ILAE diagnostic methods commission.Epilepsia. 2022 Aug;63(8):1899-1919. doi: 10.1111/epi.17301. Epub 2022 Jun 15. Epilepsia. 2022. PMID: 35706131 Free PMC article.
-
Sturge-Weber Syndrome: A Narrative Review of Clinical Presentation and Updates on Management.J Clin Med. 2025 Mar 22;14(7):2182. doi: 10.3390/jcm14072182. J Clin Med. 2025. PMID: 40217631 Free PMC article. Review.
-
Neuropathology and epilepsy surgery: 2022 update.Free Neuropathol. 2022 May 3;3:12. doi: 10.17879/freeneuropathology-2022-3813. eCollection 2022 Jan. Free Neuropathol. 2022. PMID: 37284150 Free PMC article.
-
The specific DNA methylation landscape in focal cortical dysplasia ILAE type 3D.Acta Neuropathol Commun. 2023 Aug 9;11(1):129. doi: 10.1186/s40478-023-01618-6. Acta Neuropathol Commun. 2023. PMID: 37559109 Free PMC article.
-
Pathomorphological Diagnostic Criteria for Focal Cortical Dysplasias and Other Common Epileptogenic Lesions-Review of the Literature.Diagnostics (Basel). 2023 Mar 31;13(7):1311. doi: 10.3390/diagnostics13071311. Diagnostics (Basel). 2023. PMID: 37046529 Free PMC article. Review.
References
-
- Challa VR. Vascular malformations and angiomas. In: Kalimo H, editor. Pathology & genetics. Cerebrovascular diseases. Basel: ISN Neuropath Press. 2005; p. 119–24.
-
- Blumcke I, Spreafico R, Haaker G, Coras R, Kobow K, Bien CG, et al. Histopathological findings in brain tissue obtained during epilepsy surgery. N Eng J Med. 2017;377:1648–56. - PubMed
-
- Rosenow F, Alonso‐Vanegas MA, Baumgartner C, Blümcke I, Carreño M, Gizewski ER, et al. Cavernoma‐related epilepsy: review and recommendations for management – report of the Surgical Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2013;54:2025–35. - PubMed
-
- Ivens S, Kaufer D, Flores LP, Bechmann I, Zumsteg D, Tomkins O, et al. TGF‐β receptor‐mediated albumin uptake into astrocytes is involved in neocortical epileptogenesis. Brain. 2007;130:535–47. - PubMed

