Glycemic outcomes of children 2-6 years of age with type 1 diabetes during the pediatric MiniMed™ 670G system trial
- PMID: 35001477
- PMCID: PMC9304187
- DOI: 10.1111/pedi.13312
Glycemic outcomes of children 2-6 years of age with type 1 diabetes during the pediatric MiniMed™ 670G system trial
Abstract
Background: Highly variable insulin sensitivity, susceptibility to hypoglycemia and inability to effectively communicate hypoglycemic symptoms pose significant challenges for young children with type 1 diabetes (T1D). Herein, outcomes during clinical MiniMed™ 670G system use were evaluated in children aged 2-6 years with T1D.
Methods: Participants (N = 46, aged 4.6 ± 1.4 years) at seven investigational centers used the MiniMed™ 670G system in Manual Mode during a two-week run-in period followed by Auto Mode during a three-month study phase. Safety events, mean A1C, sensor glucose (SG), and percentage of time spent in (TIR, 70-180 mg/dl), below (TBR, <70 mg/dl) and above (TAR, >180 mg/dl) range were assessed for the run-in and study phase and compared using a paired t-test or Wilcoxon signed-rank test.
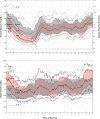
Results: From run-in to end of study (median 87.1% time in auto mode), mean A1C and SG changed from 8.0 ± 0.9% to 7.5 ± 0.6% (p < 0.001) and from 173 ± 24 to 161 ± 16 mg/dl (p < 0.001), respectively. Overall TIR increased from 55.7 ± 13.4% to 63.8 ± 9.4% (p < 0.001), while TBR and TAR decreased from 3.3 ± 2.5% to 3.2 ± 1.6% (p = 0.996) and 41.0 ± 14.7% to 33.0 ± 9.9% (p < 0.001), respectively. Overnight TBR remained unchanged and TAR was further improved 12:00 am-6:00 am. Throughout the study phase, there were no episodes of severe hypoglycemia or diabetic ketoacidosis (DKA) and no serious adverse device-related events.
Conclusions: At-home MiniMed™ 670G Auto Mode use by young children safely improved glycemic outcomes compared to two-week open-loop Manual Mode use. The improvements are similar to those observed in older children, adolescents and adults with T1D using the same system for the same duration of time.
Trial registration: ClinicalTrials.gov NCT02660827.
Keywords: A1C; automated insulin delivery; hybrid closed loop; pediatric; time-in-range; type 1 diabetes.
© 2022 The Authors. Pediatric Diabetes published by John Wiley & Sons Ltd.
Conflict of interest statement
The MiniMed™ 670G system is intended for the management of type 1 diabetes in persons 7 years of age and older.
Figures
References
-
- Writing Group for the Search for Diabetes in Youth Study Group , Dabelea D, Bell RA, et al. Incidence of diabetes in youth in the United States. JAMA. 2007;297(24):12716‐12724. - PubMed

