The role of E2A in ATPR-induced cell differentiation and cycle arrest in acute myeloid leukaemia cells
- PMID: 35001521
- PMCID: PMC8831953
- DOI: 10.1111/jcmm.17166
The role of E2A in ATPR-induced cell differentiation and cycle arrest in acute myeloid leukaemia cells
Abstract
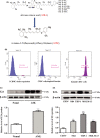
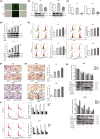
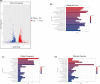
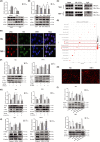
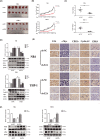
Acute myeloid leukaemia (AML) is a biologically heterogeneous disease with an overall poor prognosis; thus, novel therapeutic approaches are needed. Our previous studies showed that 4-amino-2-trifluoromethyl-phenyl retinate (ATPR), a new derivative of all-trans retinoic acid (ATRA), could induce AML cell differentiation and cycle arrest. The current study aimed to determine the potential pharmacological mechanisms of ATPR therapies against AML. Our findings showed that E2A was overexpressed in AML specimens and cell lines, and mediate AML development by inactivating the P53 pathway. The findings indicated that E2A expression and activity decreased with ATPR treatment. Furthermore, we determined that E2A inhibition could enhance the effect of ATPR-induced AML cell differentiation and cycle arrest, whereas E2A overexpression could reverse this effect, suggesting that the E2A gene plays a crucial role in AML. We identified P53 and c-Myc were downstream pathways and targets for silencing E2A cells using RNA sequencing, which are involved in the progression of AML. Taken together, these results confirmed that ATPR inhibited the expression of E2A/c-Myc, which led to the activation of the P53 pathway, and induced cell differentiation and cycle arrest in AML.
Keywords: E2A; P53 pathway; acute myeloid leukaemia; c-Myc; cycle arrest; differentiation.
© 2022 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures


References
-
- Lehrnbecher T, Varwig D, Kaiser J, Reinhardt D, Klingebiel T, Creutzig U. Infectious complications in pediatric acute myeloid leukemia: analysis of the prospective multi‐institutional clinical trial AML‐BFM 93. Leukemia. 2004;18(1):72‐77. - PubMed
-
- Just Vinholt P, Højrup Knudsen G, Sperling S, Frederiksen H, Nielsen C. Platelet function tests predict bleeding in patients with acute myeloid leukemia and thrombocytopenia. Am J Hematol. 2019;94(8):891‐901. - PubMed
-
- Siegel R, Miller K, Fuchs H, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71(1):7‐33. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

