The role of fibroblast growth factor 8 in cartilage development and disease
- PMID: 35001536
- PMCID: PMC8831980
- DOI: 10.1111/jcmm.17174
The role of fibroblast growth factor 8 in cartilage development and disease
Abstract
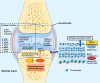
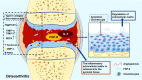
Fibroblast growth factor 8 (FGF-8), also known as androgen-induced growth factor (AIGF), is presumed to be a potent mitogenic cytokine that plays important roles in early embryonic development, brain formation and limb development. In the bone environment, FGF-8 produced or received by chondrocyte precursor cells binds to fibroblast growth factor receptor (FGFR), causing different levels of activation of downstream signalling pathways, such as phospholipase C gamma (PLCγ)/Ca2+ , RAS/mitogen-activated protein kinase-extracellular regulated protein kinases (RAS/MAPK-MEK-ERK), and Wnt-β-catenin-Axin2 signalling, and ultimately controlling chondrocyte proliferation, differentiation, cell survival and migration. However, the molecular mechanism of FGF-8 in normal or pathological cartilage remains unclear, and thus, FGF-8 represents a novel exploratory target for studies of chondrocyte development and cartilage disease progression. In this review, studies assessing the relationship between FGF-8 and chondrocytes that have been published in the past 5 years are systematically summarized to determine the probable mechanism and physiological effect of FGF-8 on chondrocytes. Based on the existing research results, a therapeutic regimen targeting FGF-8 is proposed to explore the possibility of treating chondrocyte-related diseases.
Keywords: FGF-8; cartilage; chondrocyte; osteoarthritis; skeletal system.
© 2022 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures


Similar articles
-
Fibroblast growth factor and canonical WNT/β-catenin signaling cooperate in suppression of chondrocyte differentiation in experimental models of FGFR signaling in cartilage.Biochim Biophys Acta. 2015 May;1852(5):839-50. doi: 10.1016/j.bbadis.2014.12.020. Epub 2015 Jan 2. Biochim Biophys Acta. 2015. PMID: 25558817
-
Fibroblast growth factors 2, 4, and 8 exert both negative and positive effects on limb, frontonasal, and mandibular chondrogenesis via MEK-ERK activation.J Cell Physiol. 2007 Apr;211(1):233-43. doi: 10.1002/jcp.20923. J Cell Physiol. 2007. PMID: 17167778
-
Regulation of osteoblast, chondrocyte, and osteoclast functions by fibroblast growth factor (FGF)-18 in comparison with FGF-2 and FGF-10.J Biol Chem. 2002 Mar 1;277(9):7493-500. doi: 10.1074/jbc.M108653200. Epub 2001 Dec 11. J Biol Chem. 2002. PMID: 11741978
-
Fibroblast growth factor control of cartilage homeostasis.J Cell Biochem. 2013 Apr;114(4):735-42. doi: 10.1002/jcb.24418. J Cell Biochem. 2013. PMID: 23060229 Free PMC article. Review.
-
FGF, TGFβ and Wnt crosstalk: embryonic to in vitro cartilage development from mesenchymal stem cells.J Tissue Eng Regen Med. 2015 Apr;9(4):332-42. doi: 10.1002/term.1744. Epub 2013 Apr 11. J Tissue Eng Regen Med. 2015. PMID: 23576364 Review.
Cited by
-
Signalling interaction between β-catenin and other signalling molecules during osteoarthritis development.Cell Prolif. 2024 Jun;57(6):e13600. doi: 10.1111/cpr.13600. Epub 2024 Jan 10. Cell Prolif. 2024. PMID: 38199244 Free PMC article. Review.
-
Fibroblast Growth Factors and Cellular Communication Network Factors: Intimate Interplay by the Founding Members in Cartilage.Int J Mol Sci. 2022 Aug 2;23(15):8592. doi: 10.3390/ijms23158592. Int J Mol Sci. 2022. PMID: 35955724 Free PMC article. Review.
-
Proteomic Characterization of Human Placenta: Insights into Potential Therapeutic Applications for Osteoarthritis.AAPS PharmSciTech. 2024 Jun 18;25(6):139. doi: 10.1208/s12249-024-02851-5. AAPS PharmSciTech. 2024. PMID: 38890179
-
FGF19 induces the cell cycle arrest at G2-phase in chondrocytes.Cell Death Discov. 2023 Jul 15;9(1):250. doi: 10.1038/s41420-023-01543-6. Cell Death Discov. 2023. PMID: 37454120 Free PMC article.
-
3D-printed constructs deliver bioactive cargos to expedite cartilage regeneration.J Pharm Anal. 2024 Dec;14(12):100925. doi: 10.1016/j.jpha.2023.12.015. Epub 2023 Dec 21. J Pharm Anal. 2024. PMID: 39811488 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

