Physiology and pathophysiology of human airway mucus
- PMID: 35001665
- PMCID: PMC9665957
- DOI: 10.1152/physrev.00004.2021
Physiology and pathophysiology of human airway mucus
Abstract
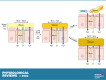
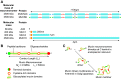
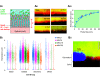
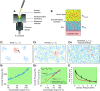
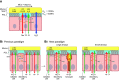
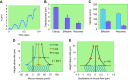
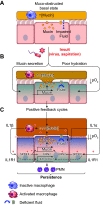
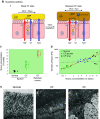
The mucus clearance system is the dominant mechanical host defense system of the human lung. Mucus is cleared from the lung by cilia and airflow, including both two-phase gas-liquid pumping and cough-dependent mechanisms, and mucus transport rates are heavily dependent on mucus concentration. Importantly, mucus transport rates are accurately predicted by the gel-on-brush model of the mucociliary apparatus from the relative osmotic moduli of the mucus and periciliary-glycocalyceal (PCL-G) layers. The fluid available to hydrate mucus is generated by transepithelial fluid transport. Feedback interactions between mucus concentrations and cilia beating, via purinergic signaling, coordinate Na+ absorptive vs Cl- secretory rates to maintain mucus hydration in health. In disease, mucus becomes hyperconcentrated (dehydrated). Multiple mechanisms derange the ion transport pathways that normally hydrate mucus in muco-obstructive lung diseases, e.g., cystic fibrosis (CF), chronic obstructive pulmonary disease (COPD), non-CF bronchiectasis (NCFB), and primary ciliary dyskinesia (PCD). A key step in muco-obstructive disease pathogenesis is the osmotic compression of the mucus layer onto the airway surface with the formation of adherent mucus plaques and plugs, particularly in distal airways. Mucus plaques create locally hypoxic conditions and produce airflow obstruction, inflammation, infection, and, ultimately, airway wall damage. Therapies to clear adherent mucus with hydrating and mucolytic agents are rational, and strategies to develop these agents are reviewed.
Keywords: airway ion transport; gel-on-brush model; mucins; muco-obstructive diseases; mucus.
Conflict of interest statement
R. C. Boucher is Chairman of the Board of Parion Sciences; is on the Scientific Advisory Board, Enterprise Therapeutics; is on the Scientific Advisory Board, Concentrix Pharma; and consults for Novartis. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
Figures














References
-
- Lucas AM, Douglas LC. Principles underlying ciliary activity in the respiratory tract: II. A comparison of nasal clearance in man, monkey and other mammals. Arch Otolaryngol 20: 518–541, 1934. doi: 10.1001/archotol.1934.03600040074006. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

