The role of junctophilin proteins in cellular function
- PMID: 35001666
- PMCID: PMC8934682
- DOI: 10.1152/physrev.00024.2021
The role of junctophilin proteins in cellular function
Abstract
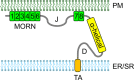
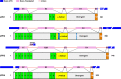
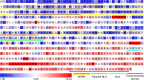
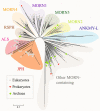
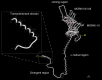
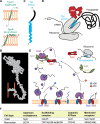
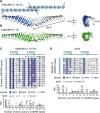
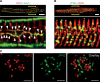
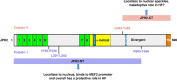
Junctophilins (JPHs) comprise a family of structural proteins that connect the plasma membrane to intracellular organelles such as the endo/sarcoplasmic reticulum (ER/SR). Tethering of these membrane structures results in the formation of highly organized subcellular junctions that play important signaling roles in all excitable cell types. There are four JPH isoforms, expressed primarily in muscle and neuronal cell types. Each JPH protein consists of six membrane occupation and recognition nexus (MORN) motifs, a joining region connecting these to another set of two MORN motifs, a putative alpha-helical region, a divergent region exhibiting low homology between JPH isoforms, and a carboxy-terminal transmembrane region anchoring into the ER/SR membrane. JPH isoforms play essential roles in developing and maintaining subcellular membrane junctions. Conversely, inherited mutations in JPH2 cause hypertrophic or dilated cardiomyopathy, while trinucleotide expansions in the JPH3 gene cause Huntington Disease-Like 2. Loss of JPH1 protein levels can cause skeletal myopathy, while loss of cardiac JPH2 levels causes heart failure and atrial fibrillation, among other disease. This review will provide a comprehensive overview of the JPH gene family, phylogeny, and evolutionary analysis of JPH genes and other MORN domain proteins. JPH biogenesis, membrane tethering, and binding partners will be discussed, as well as functional roles of JPH isoforms in excitable cells. Finally, potential roles of JPH isoform deficits in human disease pathogenesis will be reviewed.
Keywords: cardiomyopathy; excitation-contraction coupling; heart failure; junctophilins.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures




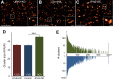

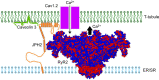
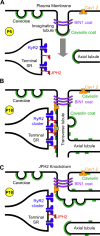
References
-
- Franzini-Armstrong C. Fine structure of sarcoplasmic reticulum and tranverse tubular system in muscle fibers. Fed Proc 23: 887–895, 1964. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

