Structural and functional properties of a magnesium transporter of the SLC11/NRAMP family
- PMID: 35001872
- PMCID: PMC8806188
- DOI: 10.7554/eLife.74589
Structural and functional properties of a magnesium transporter of the SLC11/NRAMP family
Abstract
Members of the ubiquitous SLC11/NRAMP family catalyze the uptake of divalent transition metal ions into cells. They have evolved to efficiently select these trace elements from a large pool of Ca2+ and Mg2+, which are both orders of magnitude more abundant, and to concentrate them in the cytoplasm aided by the cotransport of H+ serving as energy source. In the present study, we have characterized a member of a distant clade of the family found in prokaryotes, termed NRMTs, that were proposed to function as transporters of Mg2+. The protein transports Mg2+ and Mn2+ but not Ca2+ by a mechanism that is not coupled to H+. Structures determined by cryo-EM and X-ray crystallography revealed a generally similar protein architecture compared to classical NRAMPs, with a restructured ion binding site whose increased volume provides suitable interactions with ions that likely have retained much of their hydration shell.
Keywords: cryo-EM; isothermal titration calorimetry; magnesium transport; molecular biophysics; structural biology; transport assays; x-ray crystallogrpahy.
© 2022, Ramanadane et al.
Conflict of interest statement
KR, MS, RD, CM No competing interests declared
Figures


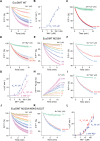

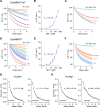

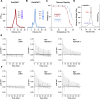
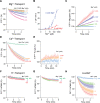



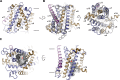

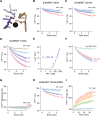

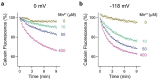
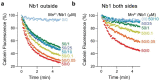
Similar articles
-
Structural and functional properties of a plant NRAMP-related aluminum transporter.Elife. 2023 Apr 19;12:e85641. doi: 10.7554/eLife.85641. Elife. 2023. PMID: 37074929 Free PMC article.
-
Structural and mechanistic basis of proton-coupled metal ion transport in the SLC11/NRAMP family.Nat Commun. 2017 Jan 6;8:14033. doi: 10.1038/ncomms14033. Nat Commun. 2017. PMID: 28059071 Free PMC article.
-
Structures in multiple conformations reveal distinct transition metal and proton pathways in an Nramp transporter.Elife. 2019 Feb 4;8:e41124. doi: 10.7554/eLife.41124. Elife. 2019. PMID: 30714568 Free PMC article.
-
Molecular Mechanism of Nramp-Family Transition Metal Transport.J Mol Biol. 2021 Aug 6;433(16):166991. doi: 10.1016/j.jmb.2021.166991. Epub 2021 Apr 16. J Mol Biol. 2021. PMID: 33865868 Free PMC article. Review.
-
Recent progress in structure-function analyses of Nramp proton-dependent metal-ion transporters.Biochem Cell Biol. 2006 Dec;84(6):960-78. doi: 10.1139/o06-193. Biochem Cell Biol. 2006. PMID: 17215883 Review.
Cited by
-
Structural and functional properties of a plant NRAMP-related aluminum transporter.Elife. 2023 Apr 19;12:e85641. doi: 10.7554/eLife.85641. Elife. 2023. PMID: 37074929 Free PMC article.
-
Antibiotic-recalcitrant Salmonella during infection.Nat Rev Microbiol. 2025 May;23(5):276-287. doi: 10.1038/s41579-024-01124-z. Epub 2024 Nov 18. Nat Rev Microbiol. 2025. PMID: 39558126 Review.
-
High-resolution structures with bound Mn2+ and Cd2+ map the metal import pathway in an Nramp transporter.Elife. 2023 Apr 11;12:e84006. doi: 10.7554/eLife.84006. Elife. 2023. PMID: 37039477 Free PMC article.
-
eGFP as an All-in-One Tag for Purification of Membrane Proteins.Methods Mol Biol. 2023;2652:171-186. doi: 10.1007/978-1-0716-3147-8_9. Methods Mol Biol. 2023. PMID: 37093475
-
Slc11 Synapomorphy: A Conserved 3D Framework Articulating Carrier Conformation Switch.Int J Mol Sci. 2023 Oct 11;24(20):15076. doi: 10.3390/ijms242015076. Int J Mol Sci. 2023. PMID: 37894758 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

