Synthesis of 2,24-Diene-12,13,15,16,34,35,37,38-octaphenyl[4.4]-triphenylparacyclophane
- PMID: 35001982
- PMCID: PMC8740886
- DOI: 10.1055/a-1479-6611
Synthesis of 2,24-Diene-12,13,15,16,34,35,37,38-octaphenyl[4.4]-triphenylparacyclophane
Abstract
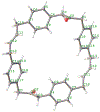
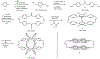
A new octaphenyl[4.4]triphenylparacyclophanediene was readily synthesized in six steps from p-xylene via the installment of bromine atoms, replacement with a vinyl group, carbonylative coupling, intermolecular followed by intramolecular double Grubbs olefin metathesis, Knoevenagel condensation, and Diels-Alder cycloaddition. The belt-shaped structure and trans-stereochemistry of the alkene moieties of the octaphenyl[4.4]triphenylparacyclophane and a synthetic intermediate, 2,21-dioxo-11,30-diene[3.4.3.4]paracyclophane, were determined by X-ray crystallography. The synthetic methodology leading to octaphenyl[4.4]triphenylparacyclophane is applicable for the synthesis of substituted triphenylparacyclophanes and possibly their corresponding bis-hexabenzocoronenylparacyclophanes via a Scholl-Mullen oxidative aryl-aryl coupling reaction.
Keywords: Diels–Alder cycloaddition; Grubbs olefin metathesis; Knoevenagel condensation; belt-shaped molecules; carbonylative coupling; cyclophanes; octaphenyl[4.4]-triphenylparacyclophane.
Conflict of interest statement
Conflict of Interest The authors declare no conflict of interest.
Figures

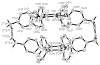
References
-
- Lehn J-M In Supramolecular Chemistry, Concepts and Perspectives; VCH: Weinheim, 1995, 1.
-
- Balzani V; Credi A; Venturi M In Molecular Devices and Machines – A Journey into the Nano World; Wiley-VCH: Weinheim, 2003, 19.
-
- van Nostrum CF; Picken SJ; Schouten A-J; Nolte RJM J. Am. Chem. Soc 1995, 117, 9957.
-
- Fokkens M; Schrader T; Klaemer F-G J. Am. Chem. Soc 2005, 127, 14415. - PubMed
-
- Kitagaki S; Shimo E; Takeda S; Fukai R; Kojima N; Yoshioka S; Takenaga N; Yoshida K Heterocycles 2021, in press, DOI: 10.3987/COM-20-S(K)58. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
