Autologous Infusion of Bone Marrow and Mesenchymal Stromal Cells in Patients with Chronic Obstructive Pulmonary Disease: Phase I Randomized Clinical Trial
- PMID: 35002228
- PMCID: PMC8733220
- DOI: 10.2147/COPD.S332613
Autologous Infusion of Bone Marrow and Mesenchymal Stromal Cells in Patients with Chronic Obstructive Pulmonary Disease: Phase I Randomized Clinical Trial
Abstract
Background and objectives: Chronic obstructive pulmonary disease (COPD) is characterized by the destruction of alveolar walls, chronic inflammation and persistent respiratory symptoms. There is no curative clinical treatment for COPD. In this context, cell-based therapy is a promising therapeutic alternative for COPD. Thus, in this open, controlled and randomized Phase I Clinical Trial, we aimed to assess the safety of the infusion of autologous bone marrow mononuclear cells (BMMC), adipose-derived mesenchymal stromal cells (ADSC) and, especially, the safety of concomitant infusion (co-infusion) of BMMC and ADSC as a new therapeutic alternative for COPD. The rationale for co-infusion of BMMC and ADSC is based on the hypothesis of an additive or synergistic therapeutic effect resulting from this association.
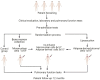
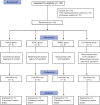
Methods: To achieve the proposed objectives, twenty patients with moderate-to-severe COPD were randomly divided into four groups: control group - patients receiving conventional treatment; BMMC group - patients receiving only BMMC; ADSC group - patients receiving only ADSC, and co-infusion group - patients receiving the concomitant infusion of BMMC and ADSC. Patients were assessed for pulmonary function, biochemical profile, and quality of life over a 12 months follow-up.
Results: No adverse events were detected immediately after the infusion of BMMC, ADSC or co-infusion. In the 12-month follow-up, no causal relationship was established between adverse events and cell therapy procedures. Regarding the efficacy, the BMMC group showed an increase in forced expiratory volume (FEV1) and diffusing capacity for carbon monoxide (DLCO). Co-infusion group showed a DLCO, and gas exchange improvement and a better quality of life.
Conclusion: The results obtained allow us to conclude that cell-based therapy with co-infusion of BMMC and ADSC is a safe procedure and a promising therapeutic for COPD. However, additional studies with a greater number of patients are needed before randomized and controlled Phase III clinical trials can be implemented.
Keywords: COPD; cell therapy; co-infusion; concomitant infusion; mesenchymal stem cells; stem cells.
© 2021 Squassoni et al.
Conflict of interest statement
All authors declare no conflicts of interest.
Figures



References
-
- Global Initiative for Chronic Obstructive Lung Disease. Global initiat chronic obstr lung disease; (2020 report); 2020. Available from: http://www.goldcopd.org/. Accessed November 2, 2021.
-
- World Health Organization. The top 10 causes of death; 2021. Available form: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death. Accessed November 2, 2021.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

