Dysregulation of the Retromer Complex in Brain Endothelial Cells Results in Accumulation of Phosphorylated Tau
- PMID: 35002279
- PMCID: PMC8721160
- DOI: 10.2147/JIR.S342096
Dysregulation of the Retromer Complex in Brain Endothelial Cells Results in Accumulation of Phosphorylated Tau
Abstract
Introduction: Transport through endothelial cells of the blood-brain barrier (BBB) involves a complex group of structures of the endo-lysosome system such as early and late endosomes, and the retromer complex system. Studies show that neuronal dysregulation of the vacuolar protein sorting 35 (VPS35), the main component of the retromer complex recognition core, results in altered protein trafficking and degradation and is involved in neurodegeneration. Since the functional role of VPS35 in endothelial cells has not been fully investigated, in the present study we aimed at characterizing the effect of its downregulation on these pathways.
Methods: Genetic silencing of VPS35 in human brain endothelial cells; measurement of retromer complex system proteins, autophagy and ubiquitin-proteasome systems.
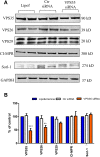
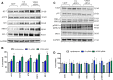
Results: VPS35-downregulated endothelial cells had increased expression of LC3B2/1 and more ubiquitinated products, markers of autophagy flux and impaired proteasome activity, respectively. Additionally, compared with controls VPS35 downregulation resulted in significant accumulation of tau protein and its phosphorylated isoforms.
Discussion: Our findings demonstrate that in brain endothelial cells retromer complex dysfunction by influencing endosome-lysosome degradation pathways results in altered proteostasis. Restoration of the retromer complex system function should be considered a novel therapeutic approach to rescue endothelial protein transport.
Keywords: Alzheimer’s disease; autophagy; brain endothelial cells; endosomal trafficking; retromer complex; tau protein; ubiquitin-proteasome.
© 2021 Filippone et al.
Conflict of interest statement
All authors declare no conflicts of interest.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

