Synthesis, characterization and in vitro, in vivo, in silico biological evaluations of substituted benzimidazole derivatives
- PMID: 35002414
- PMCID: PMC8717171
- DOI: 10.1016/j.sjbs.2021.08.082
Synthesis, characterization and in vitro, in vivo, in silico biological evaluations of substituted benzimidazole derivatives
Abstract
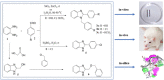
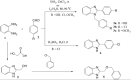
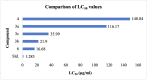
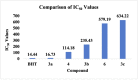
A series of substituted benzimidazole derivatives were synthesized by reacting O-phenylenediamine with various aromatic aldehydes or glycolic acid using various inexpensive reagents in aqueous media. Synthesized compounds were characterized and elucidated by IR, 1H NMR, ESI-MS spectra. Resultant compounds were screened for in vitro antimicrobial, cytotoxic, antioxidant, lipid peroxidation and cholinesterase inhibitory activities, in vivo analgesic and anti-inflammatory, and in silico anti-acetylcholinesterase and anti-butyrylcholinesterase activities. Among the synthesized compounds, compound 3b showed most promising central analgesic effect (46.15%) compared to morphine (48.08%), whereas compounds 6, 3c and 3a showed significant peripheral analgesic activity at two different dose levels (25 mg/kg and 50 mg/kg). Compounds 3b and 3a at the dose of 100 mg/kg showed significant anti-inflammatory effects from the first hour and onward, whereas compounds 6 and 3b showed moderate cytotoxic activities. In addition, compound 3a showed significant antioxidant activity having IC50 value of 16.73 µg/ml compared to 14.44 µg/ml for the standard BHT. Compound 6, 3a and 3b exhibited mild to moderate cholinesterase inhibitory activity. In silico studies revealed that compound 3a and 3b might be suitable for cholinesterase inhibitory activity. A comprehensive computational and experimental data suggested compounds 3b and 3a as the best possible candidates for pharmacological activity. All the experimental data were statistically significant (p < 0.01 level).
Keywords: Acetylcholinesterase; Anti-inflammatory; Anti-nociception; Antioxidant; Benzimidazole; Consensus Molecular Docking.
© 2021 Published by Elsevier B.V. on behalf of King Saud University.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




Similar articles
-
Synthesis, Molecular Docking, and Bioactivity Study of Novel Hybrid Benzimidazole Urea Derivatives: A Promising α-Amylase and α-Glucosidase Inhibitor Candidate with Antioxidant Activity.Pharmaceutics. 2023 Jan 30;15(2):457. doi: 10.3390/pharmaceutics15020457. Pharmaceutics. 2023. PMID: 36839780 Free PMC article.
-
Imidazole-Derived Alkyl and Aryl Ethers: Synthesis, Characterization, In Vitro Anticancer and Antioxidant Activities, Carbonic Anhydrase I-II Inhibition Properties, and In Silico Studies.ACS Omega. 2024 May 3;9(19):20937-20956. doi: 10.1021/acsomega.4c00028. eCollection 2024 May 14. ACS Omega. 2024. PMID: 38764643 Free PMC article.
-
Synthesis, Characterization and Molecular Docking Studies of Novel N-(benzimidazol-1-ylmethyl)-4-chlorobenzamide Analogues for Potential Anti-inflammatory and Antimicrobial Activity.Antiinflamm Antiallergy Agents Med Chem. 2018;17(1):16-31. doi: 10.2174/1871523017666180426125141. Antiinflamm Antiallergy Agents Med Chem. 2018. PMID: 29697033
-
Synthesis, Characterization and Cholinesterase Inhibition Studies of New Arylidene Aminothiazolylethanone Derivatives.Med Chem. 2017;13(7):648-653. doi: 10.2174/1573406413666170306113347. Med Chem. 2017. PMID: 28266279
-
Synthesis of Antimicrobial Benzimidazole-Pyrazole Compounds and Their Biological Activities.Antibiotics (Basel). 2021 Aug 19;10(8):1002. doi: 10.3390/antibiotics10081002. Antibiotics (Basel). 2021. PMID: 34439052 Free PMC article. Review.
Cited by
-
Synthesis and pharmacological evaluation of heteroarylamide derivatives as potential analgesic, anti-inflammatory, antidiarrheal and cytotoxic agents.Heliyon. 2024 Nov 22;10(23):e40630. doi: 10.1016/j.heliyon.2024.e40630. eCollection 2024 Dec 15. Heliyon. 2024. PMID: 39660188 Free PMC article.
-
Analgesic and Sedative-Hypnotic Potentiality of Crude Methanolic Extract of Gomphandra tetrandra (Wall.) Sleumer Leaves.Turk J Pharm Sci. 2022 Oct 31;19(5):583-588. doi: 10.4274/tjps.galenos.2021.09216. Turk J Pharm Sci. 2022. PMID: 36317941 Free PMC article.
-
Novel benzimidazole-based azine derivatives as potent urease inhibitors: synthesis, in vitro and in silico approach.Future Med Chem. 2024;16(22):2337-2350. doi: 10.1080/17568919.2024.2401311. Epub 2024 Sep 23. Future Med Chem. 2024. PMID: 39311079
-
A Comprehensive Account on Recent Progress in Pharmacological Activities of Benzimidazole Derivatives.Front Pharmacol. 2021 Nov 3;12:762807. doi: 10.3389/fphar.2021.762807. eCollection 2021. Front Pharmacol. 2021. PMID: 34803707 Free PMC article. Review.
-
Synthesis and toxicity of monothiooxalamides against human red blood cells, brine shrimp (Artemia salina), and fruit fly (Drosophila melanogaster).Heliyon. 2024 Aug 14;10(16):e36182. doi: 10.1016/j.heliyon.2024.e36182. eCollection 2024 Aug 30. Heliyon. 2024. PMID: 39253194 Free PMC article.
References
-
- Achar K.C., Hosamani K.M., Seetharamareddy H.R. In-vivo analgesic and anti-inflammatory activities of newly synthesized benzimidazole derivatives. Eur. J. Med. Chem. 2010;45(5):2048–2054. - PubMed
-
- Hegedüs A., Hell Z., Potor A. Zeolite-Catalyzed Environmentally Friendly Synthesis of Benzimidazole Derivatives. Synthetic Commun. 2006;36(23):3625–3630. doi: 10.1080/00397910600943865. - DOI
-
- Ahmed M., Shikha H., Sadhu S., Rahman M., Datta B. Analgesic, diuretic, and anti-inflammatory principle from Scoparia dulcis. Pharmazie. 2001;56(8):657. - PubMed
-
- Akhtar W., Khan M.F., Verma G., Shaquiquzzaman M., Rizvi M.A., Mehdi S.H., Akhter M., Alam M.M. Therapeutic evolution of benzimidazole derivatives in the last quinquennial period. Eur. J. Med. Chem. 2017;27(126):705–753. - PubMed
LinkOut - more resources
Full Text Sources

