ID09, A Newly-Designed Tubulin Inhibitor, Regulating the Proliferation, Migration, EMT Process and Apoptosis of Oral Squamous Cell Carcinoma
- PMID: 35002504
- PMCID: PMC8741845
- DOI: 10.7150/ijbs.65824
ID09, A Newly-Designed Tubulin Inhibitor, Regulating the Proliferation, Migration, EMT Process and Apoptosis of Oral Squamous Cell Carcinoma
Abstract
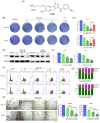
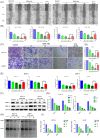
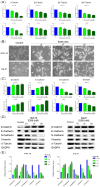
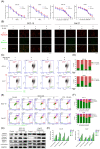
Microtubules, a major target in oral squamous cell carcinoma (OSCC) chemotherapy, contribute to multiple malignant biological behaviors, including proliferation, migration, and epithelial-mesenchymal transition (EMT). Surpassing traditional tubulin inhibitors, ID09 emerges with brilliant solubility, photostability, and drug-sensitivity in multidrug-resistant cells. Its anti-tumor effects have been briefly verified in lung adenocarcinoma and hepatocellular carcinoma. However, whether OSCC is sensitive to ID09 and the potential mechanisms remain ambiguous, which are research purposes this study aimed to achieve. Various approaches were applied, including clone formation assay, flow cytometry, wound healing assay, Transwell assay, cell counting kit-8 assay, Western blot, qRT-PCR, and in vivo experiment. The experimental results revealed that ID09 not only contributed to cell cycle arrest, reduced migration, and reversed EMT, but accelerated mitochondria-initiated apoptosis. Remarkably, Western blot detected diminishment in expression of Mcl-1 due to the deactivation of Ras-Erk pathway, resulting in ID09-induced apoptosis, proliferation and migration suppression, which could be offset by Erk1/2 phosphorylation agonist Ro 67-7476. This study initially explored the essential role Mcl-1 played and the regulatory effect of Ras-Erk pathway in anti-cancer process triggered by tubulin inhibitor, broadening clinical horizon of tubulin inhibitors in oral squamous cell carcinoma chemotherapy application.
Keywords: Apoptosis; Malignant Biological Behaviors; Mcl-1; Oral Squamous Cell Carcinoma; Ras-Erk Pathway; Tubulin Inhibitor ID09.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
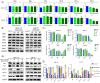
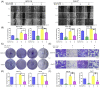
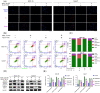
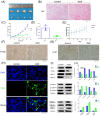
Similar articles
-
Enhancement of Histone Deacetylase Inhibitor Sensitivity in Combination with Cyclin-Dependent Kinase Inhibition for the Treatment of Oral Squamous Cell Carcinoma.Cell Physiol Biochem. 2019;53(1):141-156. doi: 10.33594/000000126. Cell Physiol Biochem. 2019. PMID: 31237760
-
Baicalein inhibits cell development, metastasis and EMT and induces apoptosis by regulating ERK signaling pathway in osteosarcoma.J Recept Signal Transduct Res. 2020 Feb;40(1):49-57. doi: 10.1080/10799893.2020.1713807. Epub 2020 Jan 17. J Recept Signal Transduct Res. 2020. PMID: 31948366
-
Oxysophocarpine Retards the Growth and Metastasis of Oral Squamous Cell Carcinoma by Targeting the Nrf2/HO-1 Axis.Cell Physiol Biochem. 2018;49(5):1717-1733. doi: 10.1159/000493615. Epub 2018 Sep 19. Cell Physiol Biochem. 2018. PMID: 30231242
-
Dietary plants for oral cancer prevention and therapy: A review of preclinical and clinical studies.Phytother Res. 2024 Nov;38(11):5225-5263. doi: 10.1002/ptr.8293. Epub 2024 Aug 28. Phytother Res. 2024. PMID: 39193857 Review.
-
Advances in Small Molecular Agents against Oral Cancer.Molecules. 2024 Apr 3;29(7):1594. doi: 10.3390/molecules29071594. Molecules. 2024. PMID: 38611874 Free PMC article. Review.
Cited by
-
Intracellular C5aR1 inhibits ferroptosis in glioblastoma through METTL3-dependent m6A methylation of GPX4.Cell Death Dis. 2024 Oct 5;15(10):729. doi: 10.1038/s41419-024-06963-5. Cell Death Dis. 2024. PMID: 39368999 Free PMC article.
-
Oleuropein regulates ubiquitination-mediated Mcl-1 turnover and exhibits antitumor activity.Cancer Gene Ther. 2025 Jul;32(7):793-805. doi: 10.1038/s41417-025-00921-9. Epub 2025 Jun 12. Cancer Gene Ther. 2025. PMID: 40506561
-
ISL1-overexpressing BMSCs attenuate renal ischemia-reperfusion injury by suppressing apoptosis and oxidative stress through the paracrine action.Cell Mol Life Sci. 2024 Jul 27;81(1):312. doi: 10.1007/s00018-024-05354-5. Cell Mol Life Sci. 2024. PMID: 39066917 Free PMC article.
-
Comprehensive RNA-seq analysis of benign prostatic hyperplasia (BPH) in rats exposed to testosterone and estradiol.Sci Rep. 2025 Jan 22;15(1):2750. doi: 10.1038/s41598-025-87205-2. Sci Rep. 2025. PMID: 39838074 Free PMC article.
-
LncRNA HOXB-AS3 promotes proliferation, migration, and invasion of gallbladder cancer cells by activating the MEK/ERK pathway.Heliyon. 2024 Aug 8;10(16):e35906. doi: 10.1016/j.heliyon.2024.e35906. eCollection 2024 Aug 30. Heliyon. 2024. PMID: 39224245 Free PMC article.
References
-
- Chi AC, Day TA, Neville BW. Oral cavity and oropharyngeal squamous cell carcinoma-an update. CA Cancer J Clin. 2015;65:401–21. - PubMed
-
- Ali J, Sabiha B, Jan HU, Haider SA, Khan AA, Ali SS. Genetic etiology of oral cancer. Oral Oncol. 2017;70:23–8. - PubMed
-
- Hussein AA, Helder MN, de Visscher JG, Leemans CR, Braakhuis BJ, de Vet HCW. et al. Global incidence of oral and oropharynx cancer in patients younger than 45 years versus older patients: A systematic review. Eur J Cancer. 2017;82:115–27. - PubMed
-
- Zhao Y, Mu X, Du G. Microtubule-stabilizing agents: New drug discovery and cancer therapy. Pharmacol Ther. 2016;162:134–43. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

