Myokine Irisin promotes osteogenesis by activating BMP/SMAD signaling via αV integrin and regulates bone mass in mice
- PMID: 35002510
- PMCID: PMC8741853
- DOI: 10.7150/ijbs.63505
Myokine Irisin promotes osteogenesis by activating BMP/SMAD signaling via αV integrin and regulates bone mass in mice
Abstract
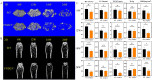
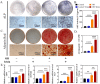
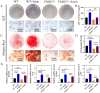
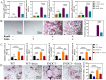
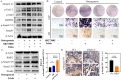
Irisin is well-known to contribute to bone homeostasis due to its bidirectional regulation on osteogenesis and osteoclastogenesis. However, the mechanisms of irisin involved in mesenchymal stem/stromal cells (MSCs)-derived osteogenesis are still under investigated. Fibronectin type III domain-containing protein 5 (FNDC5) is the precursor protein of irisin, compare with wild type (WT) littermates, FNDC5-/- mice lost bone mass significantly, collectively evidenced by the decrease of bone mineral density (BMD), impaired bone formation and reduced N-terminal propertied of type I procollagen (P1NP) in sera. Meanwhile, the bone resorbing of FNDC5-/- mice has enhanced accompanied by increased tartrate phosphatase (TRAP) staining cells morphologically and cross-Linked C-telopeptide of type 1 collagen (CTX) level in sera. In vitro study showed that lack of irisin impeded the MSC-derived osteogenesis of FNDC5-/- mice. The addition of irisin promote the osteogenesis of WT and irisin-deficient MSCs, by activating αV integrin-induced ERK/STAT pathway, subsequently enhancing bone morphogenetic protein 2 (BMP2) expression and BMP/SMAD signaling activation. Taken together, these findings further indicate that irisin regulates bone homeostasis. Moreover, irisin promotes MSC-derived osteogenesis by binding to αV integrin and activating BMP/SMAD signaling consequently. Thus, irisin may be a promising therapeutic target for osteoporosis and bone defects.
Keywords: BMP/SMAD signaling; Irisin; Osteogenesis; mesenchymal stem/stromal cell; αV integrin..
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


References
-
- Leslie WD, Morin SN. Osteoporosis epidemiology 2013: implications for diagnosis, risk assessment, and treatment. Curr Opin Rheumatol. 2014;26:440–6. - PubMed
-
- Si L, Winzenberg TM, Jiang Q, Chen M, Palmer AJ. Projection of osteoporosis-related fractures and costs in China: 2010-2050. Osteoporos Int. 2015;26:1929–37. - PubMed
-
- Levin VA, Jiang X, Kagan R. Estrogen therapy for osteoporosis in the modern era. Osteoporos Int. 2018;29:1049–55. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

