Rocaglamide promotes the infiltration and antitumor immunity of NK cells by activating cGAS-STING signaling in non-small cell lung cancer
- PMID: 35002511
- PMCID: PMC8741839
- DOI: 10.7150/ijbs.65019
Rocaglamide promotes the infiltration and antitumor immunity of NK cells by activating cGAS-STING signaling in non-small cell lung cancer
Abstract
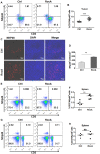
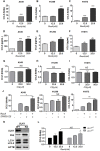
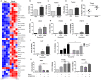
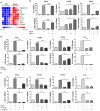
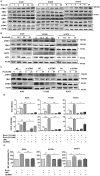
Background: Natural killer (NK) cell-based immunotherapy is clinically limited due to insufficient tumor infiltration in solid tumors. We have previously found that the natural product rocaglamide (RocA) can enhance NK cell-mediated killing of non-small cell lung cancer (NSCLC) cells by inhibiting autophagy, and autophagic inhibition has been shown to increase NK cell tumor infiltration in melanoma. Therefore, we hypothesized that RocA could increase NK cell infiltration in NSCLC by autophagy inhibition. Methods: Flow cytometry, RNA-sequencing, real-time PCR, Western blotting analysis, and xenograft tumor model were utilized to assess the infiltration of NK cells and the underlying mechanism. Results: RocA significantly increased the infiltration of NK cells and the expressions of CCL5 and CXCL10 in NSCLC cells, which could not be reversed by the inhibitions of autophagy/ULK1, JNK and NF-κB. However, such up-regulation could be suppressed by the inhibitions of TKB1 and STING. Furthermore, RocA dramatically activated the cGAS (cyclic GMP-AMP synthase)-STING (stimulator of interferon genes) signaling pathway, and the inhibition/depletion of STING ablated the up-regulation of CCL5 and CXCL10, NK cell infiltration, and tumor regression induced by RocA. Besides, RocA damaged mitochondrial DNA (mtDNA) and promoted the cytoplasmic release of mtDNA. The mPTP inhibitor cyclosporin A could reverse RocA-induced cytoplasmic release of mtDNA. Conclusions: RocA could promote NK cell infiltration by activating cGAS-STING signaling via targeting mtDNA, but not by inhibiting autophagy. Taken together, our current findings suggested that RocA was a potent cGAS-STING agonist and had a promising potential in cancer immunotherapy, especially in NK cell-based immunotherapy.
Keywords: Mitochondrial DNA; NK cells; Non-small cell lung cancer; Rocaglamide; STING.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


Similar articles
-
Rocaglamide enhances NK cell-mediated killing of non-small cell lung cancer cells by inhibiting autophagy.Autophagy. 2018;14(10):1831-1844. doi: 10.1080/15548627.2018.1489946. Epub 2018 Aug 17. Autophagy. 2018. PMID: 29969944 Free PMC article.
-
Mitochondrial DNA leakage induces odontoblast inflammation via the cGAS-STING pathway.Cell Commun Signal. 2021 May 20;19(1):58. doi: 10.1186/s12964-021-00738-7. Cell Commun Signal. 2021. PMID: 34016129 Free PMC article.
-
Selective BCL-2 inhibitor triggers STING-dependent antitumor immunity via inducing mtDNA release.J Immunother Cancer. 2025 Apr 29;13(4):e010889. doi: 10.1136/jitc-2024-010889. J Immunother Cancer. 2025. PMID: 40300857 Free PMC article.
-
cGAS-STING signaling in cancer immunity and immunotherapy.Biomed Pharmacother. 2021 Jan;133:110972. doi: 10.1016/j.biopha.2020.110972. Epub 2020 Nov 27. Biomed Pharmacother. 2021. PMID: 33254021 Review.
-
Crosstalk between cGAS-STING pathway and autophagy in cancer immunity.Front Immunol. 2023 Mar 1;14:1139595. doi: 10.3389/fimmu.2023.1139595. eCollection 2023. Front Immunol. 2023. PMID: 36936940 Free PMC article. Review.
Cited by
-
Progress of cGAS-STING signaling pathway-based modulation of immune response by traditional Chinese medicine in clinical diseases.Front Immunol. 2024 Dec 16;15:1510628. doi: 10.3389/fimmu.2024.1510628. eCollection 2024. Front Immunol. 2024. PMID: 39737190 Free PMC article. Review.
-
Regulation and Function of the cGAS-STING Pathway: Mechanisms, Post-Translational Modifications, and Therapeutic Potential in Immunotherapy.Drug Des Devel Ther. 2025 Mar 11;19:1721-1739. doi: 10.2147/DDDT.S501773. eCollection 2025. Drug Des Devel Ther. 2025. PMID: 40098909 Free PMC article. Review.
-
Clinical significance of STING expression and methylation in lung adenocarcinoma based on bioinformatics analysis.Sci Rep. 2022 Aug 17;12(1):13951. doi: 10.1038/s41598-022-18278-6. Sci Rep. 2022. PMID: 35978045 Free PMC article.
-
Understanding the molecular regulatory mechanisms of autophagy in lung disease pathogenesis.Front Immunol. 2024 Oct 31;15:1460023. doi: 10.3389/fimmu.2024.1460023. eCollection 2024. Front Immunol. 2024. PMID: 39544928 Free PMC article. Review.
-
Nanomedicines harnessing cGAS-STING pathway: sparking immune revitalization to transform 'cold' tumors into 'hot' tumors.Mol Cancer. 2024 Dec 23;23(1):277. doi: 10.1186/s12943-024-02186-6. Mol Cancer. 2024. PMID: 39710707 Free PMC article. Review.
References
-
- Crinier A, Narni-Mancinelli E, Ugolini S, Vivier E. SnapShot: Natural Killer Cells. Cell. 2020;180:1280–e1. - PubMed
-
- Halama N, Braun M, Kahlert C, Spille A, Quack C, Rahbari N. et al. Natural killer cells are scarce in colorectal carcinoma tissue despite high levels of chemokines and cytokines. Clin Cancer Res. 2011;17:678–89. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

