Trophoblast-derived Lactic Acid Orchestrates Decidual Macrophage Differentiation via SRC/LDHA Signaling in Early Pregnancy
- PMID: 35002512
- PMCID: PMC8741856
- DOI: 10.7150/ijbs.67816
Trophoblast-derived Lactic Acid Orchestrates Decidual Macrophage Differentiation via SRC/LDHA Signaling in Early Pregnancy
Abstract
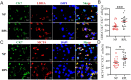
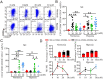
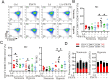
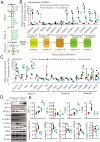
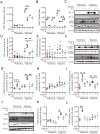
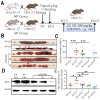
Lactic acid (LA) metabolism in the tumor microenvironment contributes to the establishment and maintenance of immune tolerance. This pathway is characterized in tumor associated macrophages. However, the role and pathway of LA metabolism at maternal-fetal interface during early pregnancy, especially in decidual macrophage differentiation, are still unclear. Herein, for the first time, we discovered that LA can trigger either M2 or M1 macrophage polarization via oxidative phosphorylation and glycolysis regulation under normoxia or hypoxia, respectively. Also, LA metabolism played a vital role in decidual macrophages-mediated recurrent pregnancy loss (RPL), through HIF-1α/SRC/LDHA pathway. Moreover, blockade of LA intake with AZD3965 (MCT-1 inhibitor) could rescue pregnancy in an abortion-prone mouse model, suggesting a potential therapeutic target in RPL. Collectively, the present study identifies the previously unknown functions of LA metabolism in the differentiation of decidual macrophages in early normal pregnancy and RPL, and provides a potential therapeutic strategy in RPL by manipulating decidual macrophages' functions through LA metabolic pathway.
Keywords: decidual macrophage; early pregnancy; lactic acid; recurrent pregnancy loss; trophoblast.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures




References
-
- Zhang Y, Ma L, Hu X, Ji J, Mor G, Liao A. The role of the PD-1/PD-L1 axis in macrophage differentiation and function during pregnancy. Hum Reprod. 2019;34:25–36. - PubMed
-
- Riera-Domingo C, Audige A, Granja S, Cheng WC, Ho PC, Baltazar F. et al. Immunity, Hypoxia, and Metabolism-the Menage a Trois of Cancer: Implications for Immunotherapy. Physiol Rev. 2020;100:1–102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

