The Different Immune Responses by Age Are due to the Ability of the Fetal Immune System to Secrete Primal Immunoglobulins Responding to Unexperienced Antigens
- PMID: 35002513
- PMCID: PMC8741860
- DOI: 10.7150/ijbs.67203
The Different Immune Responses by Age Are due to the Ability of the Fetal Immune System to Secrete Primal Immunoglobulins Responding to Unexperienced Antigens
Abstract
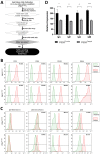
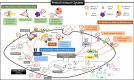
Among numerous studies on coronavirus 2019 (COVID-19), we noted that the infection and mortality rates of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) increased with age and that fetuses known to be particularly susceptible to infection were better protected despite various mutations. Hence, we established the hypothesis that a new immune system exists that forms before birth and decreases with aging. Methods: To prove this hypothesis, we established new ex-vivo culture conditions simulating the critical environmental factors of fetal stem cells (FSCs) in early pregnancy. Then, we analyzed the components from FSCs cultivated newly developed ex-vivo culture conditions and compared them from FSCs cultured in a normal condition. Results: We demonstrated that immunoglobulin M (IgM), a natural antibody (NAb) produced only in early B-1 cells, immunoglobulins (Igs) including IgG3, which has a wide range of antigen-binding capacity and affinity, complement proteins, and antiviral proteins are induced in FSCs only cultured in newly developed ex-vivo culture conditions. Particularly we confirmed that their extracellular vesicles (EVs) contained NAbs, Igs, various complement proteins, and antiviral proteins, as well as human leukocyte antigen G (HLA-G), responsible for immune tolerance. Conclusion: Our results suggest that FSCs in early pregnancy can form an independent immune system responding to unlearned antigens as a self-defense mechanism before establishing mature immune systems. Moreover, we propose the possibility of new solutions to cope with various infectious diseases based on the factors in NAbs-containing EVs, especially not causing unnecessary immune reaction due to HLA-G.
Keywords: IgG3; SARS-CoV-2; extracellular vesicles; fetal immune system; natural antibody.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures






Similar articles
-
Perspective of HLA-G Induced Immunosuppression in SARS-CoV-2 Infection.Front Immunol. 2021 Dec 6;12:788769. doi: 10.3389/fimmu.2021.788769. eCollection 2021. Front Immunol. 2021. PMID: 34938296 Free PMC article. Review.
-
Study of immune-tolerized cell lines and extracellular vesicles inductive environment promoting continuous expression and secretion of HLA-G from semiallograft immune tolerance during pregnancy.J Extracell Vesicles. 2020 Jul 20;9(1):1795364. doi: 10.1080/20013078.2020.1795364. J Extracell Vesicles. 2020. PMID: 32944184 Free PMC article.
-
SARS-CoV-2 Antibodies Mediate Complement and Cellular Driven Inflammation.Front Immunol. 2021 Nov 2;12:767981. doi: 10.3389/fimmu.2021.767981. eCollection 2021. Front Immunol. 2021. PMID: 34804055 Free PMC article.
-
Pre-COVID-19 Immunity to Common Cold Human Coronaviruses Induces a Recall-Type IgG Response to SARS-CoV-2 Antigens Without Cross-Neutralisation.Front Immunol. 2022 Feb 11;13:790334. doi: 10.3389/fimmu.2022.790334. eCollection 2022. Front Immunol. 2022. PMID: 35222375 Free PMC article.
-
Insight into the pediatric and adult dichotomy of COVID-19: Age-related differences in the immune response to SARS-CoV-2 infection.Pediatr Pulmonol. 2020 Oct;55(10):2556-2564. doi: 10.1002/ppul.24981. Epub 2020 Aug 4. Pediatr Pulmonol. 2020. PMID: 32710693 Review.
Cited by
-
Immune Tolerance vs. Immune Resistance: The Interaction Between Host and Pathogens in Infectious Diseases.Front Vet Sci. 2022 Mar 29;9:827407. doi: 10.3389/fvets.2022.827407. eCollection 2022. Front Vet Sci. 2022. PMID: 35425833 Free PMC article.
-
Inhibition of porcine deltacoronavirus entry and replication by Cepharanthine.Virus Res. 2024 Feb;340:199303. doi: 10.1016/j.virusres.2023.199303. Epub 2023 Dec 30. Virus Res. 2024. PMID: 38145807 Free PMC article.
References
-
- CDC. Long-Term Effects of COVID-19. Revised 21 January 2021. https://www.cdc.gov/
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

