A systematic review of Vaccine Breakthrough Infections by SARS-CoV-2 Delta Variant
- PMID: 35002532
- PMCID: PMC8741840
- DOI: 10.7150/ijbs.68973
A systematic review of Vaccine Breakthrough Infections by SARS-CoV-2 Delta Variant
Abstract
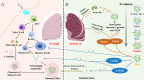
Vaccines are proving to be highly effective in controlling hospitalization and deaths associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, as shown by clinical trials and real-world evidence. However, a deadly second wave of coronavirus disease 2019 (COVID-19), infected by SARS-CoV-2 variants, especially the Delta (B.1.617.2) variant, with an increased number of post-vaccination breakthrough infections were reported in the world recently. Actually, Delta variant not only resulted in a severe surge of vaccine breakthrough infections which was accompanied with high viral load and transmissibility, but also challenged the development of effective vaccines. Therefore, the biological characteristics and epidemiological profile of Delta variant, the current status of Delta variant vaccine breakthrough infections and the mechanism of vaccine breakthrough infections were discussed in this article. In addition, the significant role of the Delta variant spike (S) protein in the mechanism of immune escape of SARS-CoV-2 was highlighted in this article. In particular, we further discussed key points on the future SARS-CoV-2 vaccine research and development, hoping to make a contribution to the early, accurate and rapid control of the COVID-19 epidemic.
Keywords: Breakthrough infection; COVID-19; Delta variant; S protein; SARS-CoV-2; Vaccine.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
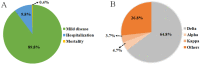
References
-
- Mlcochova P, Kemp S, Dhar MS, Papa G, Meng B, Mishra S, et al. SARS-CoV-2 B.1.617.2 Delta variant emergence and vaccine breakthrough. bioRxiv. 2021: 2021.05.08.443253.
-
- Arora P, Sidarovich A, Krüger N, Graichen L, Moldenhauer A, Winkler MS, et al. Increased lung cell entry of B.1.617.2 and evasion of antibodies induced by infection and BNT162b2. bioRxiv. 2021: 2021.06.23.449568.
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

