Extended Interval Aminoglycoside Treatment for Klebsiella Pneumoniae Endocarditis in an Extremely Low Birth Weight Neonate
- PMID: 35002564
- PMCID: PMC8717611
- DOI: 10.5863/1551-6776-27.1.85
Extended Interval Aminoglycoside Treatment for Klebsiella Pneumoniae Endocarditis in an Extremely Low Birth Weight Neonate
Abstract
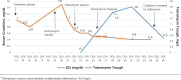
Infective endocarditis (IE) in neonates is associated with high mortality and incidence has been increasing over the past two decades. The majority of very low birth weight infants will be treated with at least one nephrotoxic medication during their hospital course. Over one-quarter of very low birth weight neonates exposed to gentamicin may develop acute kidney injury (AKI); this is particularly worrisome as AKI is an independent factor associated with increased neonatal mortality and increased length of stay. AKI during periods of neonatal nephrogenesis, which continues until 34-36 weeks postmenstrual age, may also have serious effects on the long-term nephron development which subsequently puts infants at risk of chronic kidney disease. Extended interval (EI) aminoglycoside (AMG) dosing has been used for decades in adult populations and has proven to reduce AKI while being at least as effective as traditional dosing, although there is limited published research for using an EI AMG in endocarditis in adults or pediatric patients. We describe an extremely low birth weight neonate, born preterm at 24 weeks gestation treated for Klebsiella pneumoniae IE that required AMG therapy who also had concurrent AKI. We utilized EI AMG combination therapy for treatment of Klebsiella pneumoniae endocarditis with good outcome and encourage others to report their experiences to improve our knowledge of EI AMG in this population.
Keywords: Klebsiella pneumoniae; acute kidney injury; aminoglycoside; bacterial endocarditis; extended interval; tobramycin.
Copyright. Pediatric Pharmacy Association. All rights reserved. For permissions, email: mhelms@pediatricpharmacy.org 2022.
Conflict of interest statement
Disclosures. The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria. The authors had full access to all patient information in this report and take responsibility for the integrity and accuracy of the report.
Figures
Similar articles
-
Acute kidney injury in neonatal intensive care: Medicines involved.Int J Risk Saf Med. 2015;27 Suppl 1:S9-S10. doi: 10.3233/JRS-150669. Int J Risk Saf Med. 2015. PMID: 26639729
-
Incidence, risk factors, and outcome of neonatal acute kidney injury: a prospective cohort study.Pediatr Nephrol. 2018 Sep;33(9):1617-1624. doi: 10.1007/s00467-018-3966-7. Epub 2018 Jun 5. Pediatr Nephrol. 2018. PMID: 29869723
-
Management of Acute Kidney Injury in Extremely Low Birth Weight Infants.Front Pediatr. 2022 Mar 30;10:867715. doi: 10.3389/fped.2022.867715. eCollection 2022. Front Pediatr. 2022. PMID: 35433560 Free PMC article. Review.
-
Evaluating the incidence of acute kidney injury and gentamicin synergy dosing for endocarditis.JAC Antimicrob Resist. 2023 Dec 28;6(1):dlad144. doi: 10.1093/jacamr/dlad144. eCollection 2024 Feb. JAC Antimicrob Resist. 2023. PMID: 38161960 Free PMC article.
-
Maternal and environmental risk factors for neonatal AKI and its long-term consequences.Nat Rev Nephrol. 2018 Nov;14(11):688-703. doi: 10.1038/s41581-018-0054-y. Nat Rev Nephrol. 2018. PMID: 30224767 Review.
Cited by
-
Infective Endocarditis in High-Income Countries.Metabolites. 2022 Jul 25;12(8):682. doi: 10.3390/metabo12080682. Metabolites. 2022. PMID: 35893249 Free PMC article. Review.
References
-
- Ferrieri P, Gewitz MH, Gerber MA et al. Unique features of infective endocarditis in childhood. Circulation . 2002;105(17):2115–2126. - PubMed
-
- Baltimore RS, Gewitz M, Baddour LM et al. Infective Endocarditis in Childhood: 2015 Update: A Scientific Statement From the American Heart Association. Circulation . 2015;132(15):1487–1515. - PubMed
-
- Day MD, Gauvreau K, Shulman S, Newburger JW. Characteristics of children hospitalized with infective endocarditis [published correction appears in Circulation. 2010;122:e560] Circulation . 2009;119:865–870. - PubMed
-
- Smyth A, Tan KHV, Hyman-Taylor P et al. Once versus three-times daily regimens of tobramycin treatment for pulmonary exacerbations of cystic fibrosis—the TOPIC study: a randomised controlled trial. Lancet . 2005;365(9459):573–578. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous