Neuropeptide S Attenuates the Alarm Pheromone-Evoked Defensive and Risk Assessment Behaviors Through Activation of Cognate Receptor-Expressing Neurons in the Posterior Medial Amygdala
- PMID: 35002616
- PMCID: PMC8739225
- DOI: 10.3389/fnmol.2021.752516
Neuropeptide S Attenuates the Alarm Pheromone-Evoked Defensive and Risk Assessment Behaviors Through Activation of Cognate Receptor-Expressing Neurons in the Posterior Medial Amygdala
Abstract
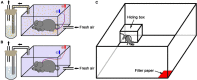
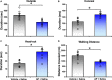
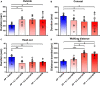
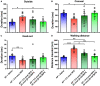
Neuropeptide S (NPS) acts by activating its cognate receptor (NPSR). High level expression of NPSR in the posterior medial amygdala suggests that NPS-NPSR system should be involved in regulation of social behaviors induced by social pheromones. The present study was undertaken to investigate the effects of central administration of NPS or with NPSR antagonist on the alarm pheromone (AP)-evoked defensive and risk assessment behaviors in mice. Furthermore, H129-H8, a novel high-brightness anterograde multiple trans-synaptic virus, c-Fos and NPSR immunostaining were employed to reveal the involved neurocircuits and targets of NPS action. The mice exposed to AP displayed an enhancement in defensive and risk assessment behaviors. NPS (0.1-1 nmol) intracerebroventricular (i.c.v.) injection significantly attenuated the AP-evoked defensive and risk assessment behaviors. NPSR antagonist [D-Val5]NPS at the dose of 40 nmol completely blocked the effect of 0.5 nmol of NPS which showed the best effective among dose range. The H129-H8-labeled neurons were observed in the bilateral posterodorsal medial amygdala (MePD) and posteroventral medial amygdala (MePV) 72 h after the virus injection into the unilateral olfactory bulb (OB), suggesting that the MePD and MePV receive olfactory information inputs from the OB. The percentage of H129-H8-labeled neurons that also express NPSR were 90.27 ± 3.56% and 91.67 ± 2.46% in the MePD and MePV, respectively. NPS (0.5 nmol, i.c.v.) remarkably increased the number of Fos immunoreactive (-ir) neurons in the MePD and MePV, and the majority of NPS-induced Fos-ir neurons also expressed NPSR. The behavior characteristic of NPS or with [D-Val5]NPS can be better replicated in MePD/MePV local injection within lower dose. The present findings demonstrated that NPS, via selective activation of the neurons bearing NPSR in the posterior medial amygdala, attenuates the AP-evoked defensive and risk assessment behaviors in mice.
Keywords: alarm pheromone; antagonist; c-Fos; herpes simplex virus; neural circuit tracing; neuropeptide S; neuropeptide S receptor; posterior medial amygdala.
Copyright © 2021 Shao, Wang, Rao, Wang, Ren, Li, Dong, Xie, Yang, Xu and Hou.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Neuropeptide S facilitates mice olfactory function through activation of cognate receptor-expressing neurons in the olfactory cortex.PLoS One. 2013 Apr 16;8(4):e62089. doi: 10.1371/journal.pone.0062089. Print 2013. PLoS One. 2013. PMID: 23614017 Free PMC article.
-
Neuropeptide S ameliorates olfactory spatial memory impairment induced by scopolamine and MK801 through activation of cognate receptor-expressing neurons in the subiculum complex.Brain Struct Funct. 2016 Jul;221(6):3327-36. doi: 10.1007/s00429-015-1103-y. Epub 2015 Sep 1. Brain Struct Funct. 2016. PMID: 26323488
-
Sex differences in olfactory-induced neural activation of the amygdala.Behav Brain Res. 2018 Jul 2;346:96-104. doi: 10.1016/j.bbr.2017.11.034. Epub 2017 Dec 2. Behav Brain Res. 2018. PMID: 29203334
-
Identification of Small Molecule Antagonists of the Neuropeptide-S Receptor.2010 Mar 19 [updated 2010 Dec 16]. In: Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010–. 2010 Mar 19 [updated 2010 Dec 16]. In: Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2010–. PMID: 21433397 Free Books & Documents. Review.
-
Morphological and functional features of the sex steroid-responsive posterodorsal medial amygdala of adult rats.Mini Rev Med Chem. 2012 Oct;12(11):1090-106. doi: 10.2174/138955712802762211. Mini Rev Med Chem. 2012. PMID: 22827219 Review.
Cited by
-
Stereotaxic atlas of the infant rat brain at postnatal days 7-13.Front Neuroanat. 2022 Aug 12;16:968320. doi: 10.3389/fnana.2022.968320. eCollection 2022. Front Neuroanat. 2022. PMID: 36032994 Free PMC article.
-
The role of ciliopathy-associated type 3 adenylyl cyclase in infanticidal behavior in virgin adult male mice.iScience. 2022 Jun 4;25(7):104534. doi: 10.1016/j.isci.2022.104534. eCollection 2022 Jul 15. iScience. 2022. PMID: 35754726 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

