Nano-Organization at the Synapse: Segregation of Distinct Forms of Neurotransmission
- PMID: 35002671
- PMCID: PMC8727373
- DOI: 10.3389/fnsyn.2021.796498
Nano-Organization at the Synapse: Segregation of Distinct Forms of Neurotransmission
Abstract
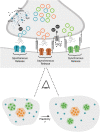
Synapses maintain synchronous, asynchronous, and spontaneous modes of neurotransmission through distinct molecular and biochemical pathways. Traditionally a single synapse was assumed to have a homogeneous organization of molecular components both at the active zone and post-synaptically. However, recent advancements in experimental tools and the further elucidation of the physiological significance of distinct forms of release have challenged this notion. In comparison to rapid evoked release, the physiological significance of both spontaneous and asynchronous neurotransmission has only recently been considered in parallel with synaptic structural organization. Active zone nanostructure aligns with postsynaptic nanostructure creating a precise trans-synaptic alignment of release sites and receptors shaping synaptic efficacy, determining neurotransmission reliability, and tuning plasticity. This review will discuss how studies delineating synaptic nanostructure create a picture of a molecularly heterogeneous active zone tuned to distinct forms of release that may dictate diverse synaptic functional outputs.
Keywords: asynchronous neurotransmission; nanocolumn; spontaneous neurotransmission; synaptic transmission and plasticity; synchronous neurotransmission.
Copyright © 2021 Guzikowski and Kavalali.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

