Snake Venom Proteomics of Samar Cobra (Naja samarensis) from the Southern Philippines: Short Alpha-Neurotoxins as the Dominant Lethal Component Weakly Cross-Neutralized by the Philippine Cobra Antivenom
- PMID: 35002690
- PMCID: PMC8740184
- DOI: 10.3389/fphar.2021.727756
Snake Venom Proteomics of Samar Cobra (Naja samarensis) from the Southern Philippines: Short Alpha-Neurotoxins as the Dominant Lethal Component Weakly Cross-Neutralized by the Philippine Cobra Antivenom
Abstract
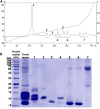
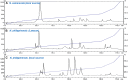
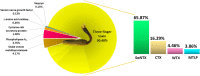
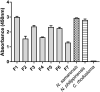
The Samar Cobra, Naja samarensis, is endemic to the southern Philippines and is a WHO-listed Category 1 venomous snake species of medical importance. Envenomation caused by N. samarensis results in neurotoxicity, while there is no species-specific antivenom available for its treatment. The composition and neutralization of N. samarensis venom remain largely unknown to date. This study thus aimed to investigate the venom proteome of N. samarensis for a comprehensive profiling of the venom composition, and to examine the immunorecognition as well as neutralization of its toxins by a hetero-specific antivenom. Applying C18 reverse-phase high-performance liquid chromatography (RP-HPLC) and tandem mass spectrometry (LC-MS/MS), three-finger toxins (3FTx) were shown to dominate the venom proteome by 90.48% of total venom proteins. Other proteins in the venom comprised snake venom metalloproteinases, phospholipases A2, cysteine-rich secretory proteins, venom nerve growth factors, L-amino acid oxidases and vespryn, which were present at much lower abundances. Among all, short-chain alpha-neurotoxins (SαNTX) were the most highly expressed toxin within 3FTx family, constituting 65.87% of the total venom proteins. The SαNTX is the sole neurotoxic component of the venom and has an intravenous median lethal dose (LD50) of 0.18 μg/g in mice. The high abundance and low LD50 support the potent lethal activity of N. samarensis venom. The hetero-specific antivenom, Philippine Cobra Antivenom (PCAV, raised against Naja philippinensis) were immunoreactive toward the venom and its protein fractions, including the principal SαNTX. In efficacy study, PCAV was able to cross-neutralize the lethality of SαNTX albeit the effect was weak with a low potency of 0.20 mg/ml (defined as the amount of toxin completely neutralized per milliliter of the antivenom). With a volume of 5 ml, each vial of PCAV may cross-neutralize approximately 1 mg of the toxin in vivo. The findings support the potential para-specific use of PCAV in treating envenomation caused by N. samarensis while underscoring the need to improve the potency of its neutralization activity, especially against the highly lethal alpha-neurotoxins.
Keywords: Southern Philippine Cobra; alpha-neurotoxin; immunoreactivity; spitting cobra; venomics.
Copyright © 2021 Palasuberniam, Chan, Tan and Tan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Proteomic insights into short neurotoxin-driven, highly neurotoxic venom of Philippine cobra (Naja philippinensis) and toxicity correlation of cobra envenomation in Asia.J Proteomics. 2019 Aug 30;206:103418. doi: 10.1016/j.jprot.2019.103418. Epub 2019 Jun 12. J Proteomics. 2019. PMID: 31201947
-
Immunoreactivity and neutralization capacity of Philippine cobra antivenom against Naja philippinensis and Naja samarensis venoms.Trans R Soc Trop Med Hyg. 2021 Jan 7;115(1):78-84. doi: 10.1093/trstmh/traa087. Trans R Soc Trop Med Hyg. 2021. PMID: 32945886
-
Venomics of Naja sputatrix, the Javan spitting cobra: A short neurotoxin-driven venom needing improved antivenom neutralization.J Proteomics. 2017 Mar 22;157:18-32. doi: 10.1016/j.jprot.2017.01.018. Epub 2017 Jan 31. J Proteomics. 2017. PMID: 28159706
-
King Cobra and snakebite envenomation: on the natural history, human-snake relationship and medical importance of Ophiophagus hannah.J Venom Anim Toxins Incl Trop Dis. 2022 Jan 5;27:e20210051. doi: 10.1590/1678-9199-JVATITD-2021-0051. eCollection 2021. J Venom Anim Toxins Incl Trop Dis. 2022. PMID: 35069710 Free PMC article. Review.
-
Mass spectrometric analysis to unravel the venom proteome composition of Indian snakes: opening new avenues in clinical research.Expert Rev Proteomics. 2020 May;17(5):411-423. doi: 10.1080/14789450.2020.1778471. Epub 2020 Jun 24. Expert Rev Proteomics. 2020. PMID: 32579411 Review.
Cited by
-
Editorial: Venoms and Toxins: Functional Omics and Pharmacological Insights.Front Pharmacol. 2022 Apr 27;13:887513. doi: 10.3389/fphar.2022.887513. eCollection 2022. Front Pharmacol. 2022. PMID: 35571136 Free PMC article. No abstract available.
-
Adding insult to injury: A review of infections following envenomings.Toxicon X. 2025 Jun 23;27:100230. doi: 10.1016/j.toxcx.2025.100230. eCollection 2025 Sep. Toxicon X. 2025. PMID: 40678361 Free PMC article. Review.
-
Isolation and Characterization of Two Postsynaptic Neurotoxins From Indian Cobra (Naja Naja) Venom.Front Pharmacol. 2022 Mar 28;13:815079. doi: 10.3389/fphar.2022.815079. eCollection 2022. Front Pharmacol. 2022. PMID: 35418867 Free PMC article.
-
Equatorial Spitting Cobra (Naja sumatrana) from Malaysia (Negeri Sembilan and Penang), Southern Thailand, and Sumatra: Comparative Venom Proteomics, Immunoreactivity and Cross-Neutralization by Antivenom.Toxins (Basel). 2022 Jul 29;14(8):522. doi: 10.3390/toxins14080522. Toxins (Basel). 2022. PMID: 36006183 Free PMC article.
-
Kaempferol mitigates reproductive dysfunctions induced by Naja nigricollis venom through antioxidant system and anti-inflammatory response in male rats.Sci Rep. 2024 Feb 16;14(1):3933. doi: 10.1038/s41598-024-54523-w. Sci Rep. 2024. PMID: 38365877 Free PMC article.
References
-
- Avilonzoo. (2015). Samar Cobra. Available at: https://www.avilonzoo.ph/avilon-zoo/meet-our-animals/item/65-samar-cobra . [Accessed May 21, 2021].
-
- Chanda A., Patra A., Kalita B., Mukherjee A. K. (2018). Proteomics Analysis to Compare the Venom Composition between Naja naja and Naja kaouthia from the Same Geographical Location of Eastern India: Correlation with Pathophysiology of Envenomation and Immunological Cross-Reactivity towards Commercial Polyantivenom. Expert Rev. Proteomics 15 (11), 949–961. 10.1080/14789450.2018.1538799 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

